题目
CHM1052 - MUM S2 2025 CHM1052 Ex 3 - Fischer esterification (Benzocaine) - Pre-lab exercise
多项填空题
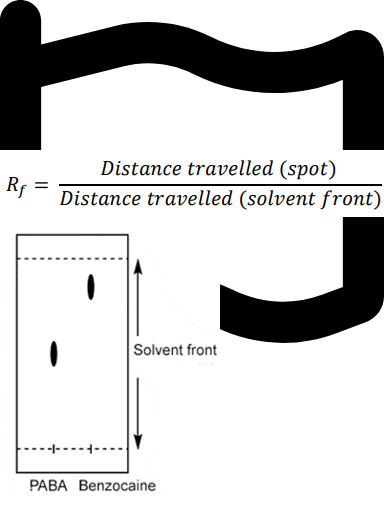
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
查看解析
标准答案
Please login to view
思路分析
First, I will restate the problem details to establish the data we’re working with: distances travelled by the compounds are PABA = 2.1 cm, Benzocaine = 3.5 cm, and the solvent front distance = 4.1 cm. The TLC formula for the retention factor is Rf = (Distance travelled by the compound) / (Distance travelled by the solvent f......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Question textAn example of Thin Layer Chromatography (TLC) results:Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate.Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures)Please refer to p.46 in the lab manual for more information.[table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cmBenzocaine: Distance travelled: 3.5 cmSolvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table]Based on the information above, which compound is less polar? Answer 3 Question 6[select: , Benzocaine, PABA, Neither]
Q6 V3Consider the following TLC plate of compounds X, Y and Z developed using a suitable mobile phase on a polar stationary phase. Which of the following is correct?
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Samples of three amino acids A, B and C, were spotted on the base line of a TLC plate. The chromatogram produced using a particular solvent is shown below: Amino acids Rf values alanine 0.56 isoleucine 0.79 taurine 0.34 Use the TLC plate and Rf values given to identify amino acid A.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!