题目
MUF0042 Chemistry Unit 2 - Semester 2, 2025
单项选择题
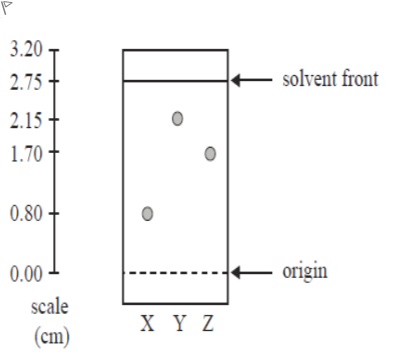
Q6 V3Consider the following TLC plate of compounds X, Y and Z developed using a suitable mobile phase on a polar stationary phase. Which of the following is correct?
选项
A.a. Z is more polar than X and has a higher Rf value than X
B.b. X is the most polar component and has an Rf value of 0.80
C.c. Z is more polar than Y and has a higher Rf value than Y
D.d. Y is the least polar component and has an Rf value of 0.78
查看解析
标准答案
Please login to view
思路分析
Question restatement: The TLC plate shows three components X, Y and Z developed on a polar stationary phase with a suitable mobile phase. We must choose which statement is correct about their relative polarity and Rf values.
Option a: 'Z is more polar than X and has a higher Rf value than X.' On a polar stationary phase, more polar compounds interact more strongly with the stationary phase, which lowers their Rf (they travel less). If Z were more polar than X, Z would typically have a lower Rf, n......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Question textAn example of Thin Layer Chromatography (TLC) results:Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate.Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures)Please refer to p.46 in the lab manual for more information.[table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cmBenzocaine: Distance travelled: 3.5 cmSolvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table]Based on the information above, which compound is less polar? Answer 3 Question 6[select: , Benzocaine, PABA, Neither]
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Samples of three amino acids A, B and C, were spotted on the base line of a TLC plate. The chromatogram produced using a particular solvent is shown below: Amino acids Rf values alanine 0.56 isoleucine 0.79 taurine 0.34 Use the TLC plate and Rf values given to identify amino acid A.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!