题目
SPHY001 Practice Quiz - Unit 15 | LA055
单项选择题
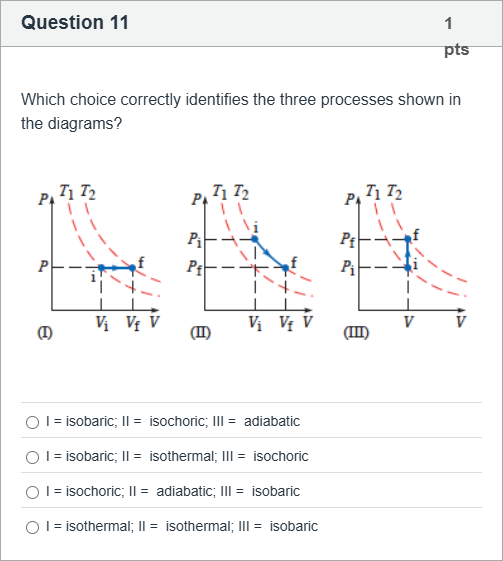
Which choice correctly identifies the three processes shown in the diagrams?
选项
A.I = isobaric; II = isochoric; III = adiabatic
B.I = isobaric; II = isothermal; III = isochoric
C.I = isochoric; II = adiabatic; III = isobaric
D.I = isothermal; II = isothermal; III = isobaric
查看解析
标准答案
Please login to view
思路分析
First, we list the given answer options and restate the question clearly: Which choice correctly identifies the three processes shown in the diagrams? I, II, and III correspond to the three processes, and the options assign each letter to a specific process (isobaric, isochoric, isothermal, adiabatic).
Option A: I = isobaric; II = isochoric; III = adiabatic
This option claims II is isochoric (constant volume) and III is adiabatic (temperature change with no heat transfer). If the diagram for II actually shows a vertical movement at constant volume, that would align with isochoric; however, the typical isochoric P–V path is vertical, not horizontal, and the third diagram would need to reflect adiabatic behavior (P V^γ = constant with T changing). Without seeing exact curves, this option risks mislabeling the second and third processes if th......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
In Stop to Think 19.2, which process results in the gas having a larger final temperature when the gas reaches State 3?
The image below shows a p-V diagram with isotherms indicated in blue. Match each of the marked paths A, B, C, D with the corresponding thermodynamic processes.
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!