题目
PHAS0006_24-25 Quiz for week 1
匹配题
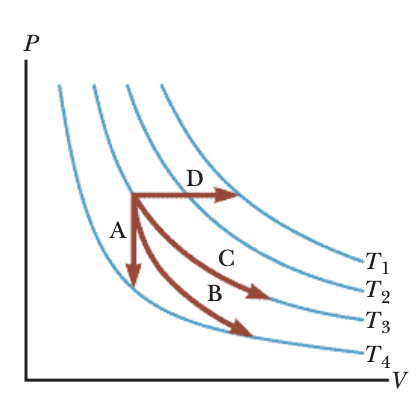
The image below shows a p-V diagram with isotherms indicated in blue. Match each of the marked paths A, B, C, D with the corresponding thermodynamic processes.
选项
A.Isobaric
B.Isothermal
C.Isosceles
D.Isovolumetric
E.Adiabatic
查看解析
标准答案
Please login to view
思路分析
Let's break down what each labeled path represents on a p–V diagram with the blue curves as isotherms.
A: The path A is drawn as a vertical segment, meaning the volume does not change while pressure changes. A vertical process on a p–V diagram corresponds to an isovolumetric (or isochoric) process. Since volume is fixed, heat transfer changes pressure, but volume remains constant. This matches the concept of isovolumetric.
B: The path B travels downward and to the right, moving to larger volume with decreasing pressure, along a traject......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which choice correctly identifies the three processes shown in the diagrams?
In Stop to Think 19.2, which process results in the gas having a larger final temperature when the gas reaches State 3?
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!