题目
单项选择题
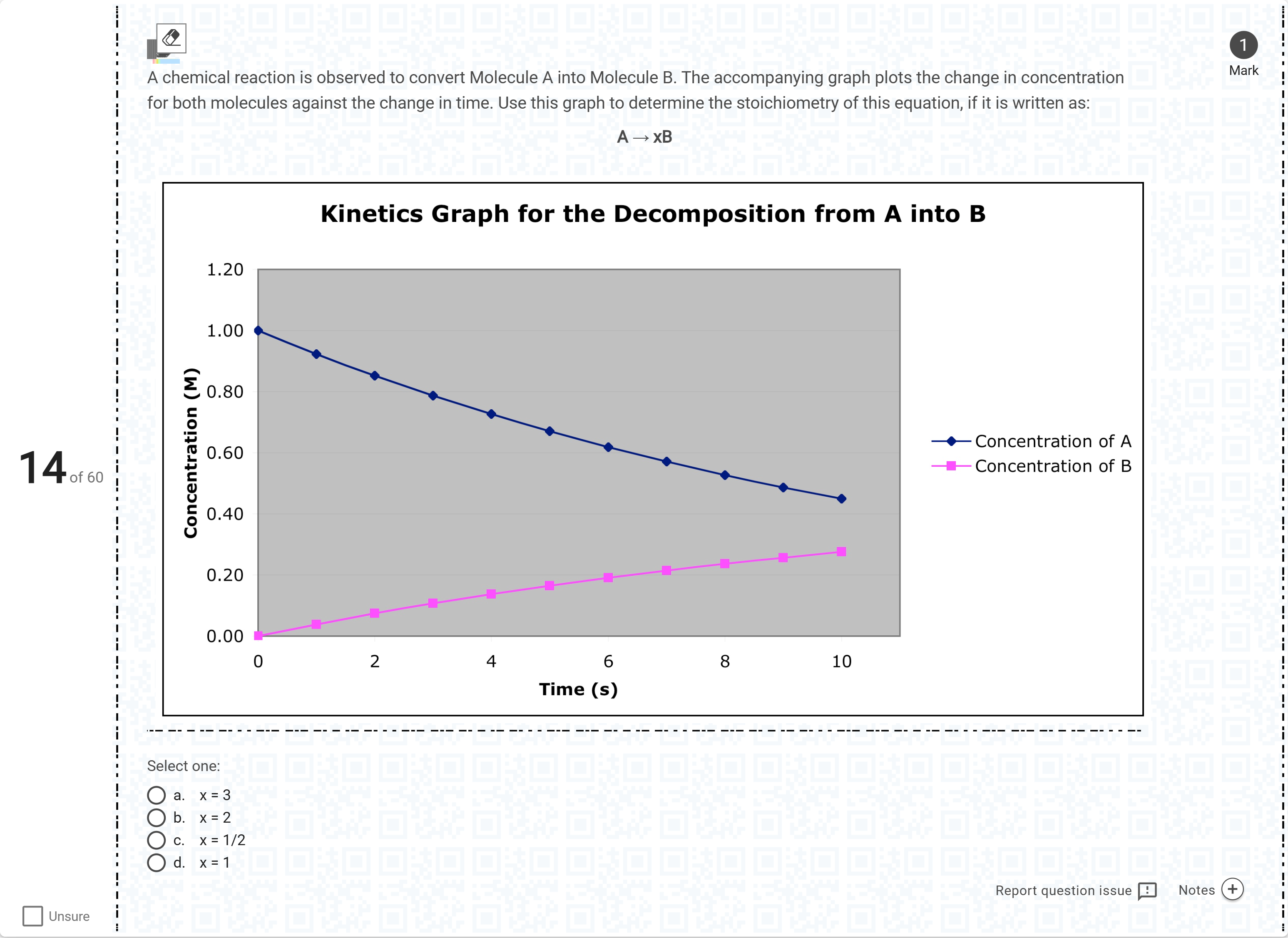
A chemical reaction is observed to convert Molecule A into Molecule B. The accompanying graph plots the change in concentration for both molecules against the change in time. Use this graph to determine the stoichiometry of this equation, if it is written as: A → xB[Fill in the blank]
选项
A.a. x = 3
B.b. x = 2
C.c. x = 1/2
D.d. x = 1
查看解析
标准答案
Please login to view
思路分析
Begin by restating the given information from the graph: A is consumed over time while B is formed, and the stoichiometry is written as A → x B.
Option a (x = 3): If x were 3, then for every 1 mole of A consumed, 3 moles of B should be produced. Checking the graph, the amount of A decreases by about 0.55 M while B increases by only abo......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following diagrams illustrates the law of multiple proportions?
If sodium hydrogen carbonate is added to a hydrochloric acid solution, it will produce gaseous carbon dioxide according to the following reaction: NaHCO3(s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g) How many grams of NaHCO3 must be added to an excess of HCl(aq) to produce 50.0 mL of CO2 at 25 °C and 0.995 atm?
Carbon monoxide and oxygen react to form carbon dioxide according to the following balanced chemical equation 2 CO(g) + O2(g) ➔ 2 CO2(g) If CO(g) and O2(g) are combined in the ratio shown in the diagram below, where the black spheres represent carbon atoms and the red spheres represent oxygen atoms, which molecules will remain when the reaction is complete?
Consider the following reaction: 4FeS2(s) + 11O2(g) → 2Fe2O3(s) + 8SO2(g) If 4.0 mol of FeS2 and 4.0 mol of O2 react, how many moles of Fe2O3(s) are produced?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!