题目
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 21 Quiz C09-3
多重下拉选择题
How many sigma and pi bonds are in the molecule shown below? Sigma bonds: 8 Pi bonds: 1
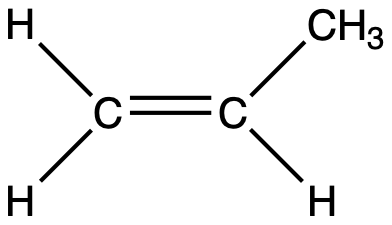
查看解析
标准答案
Please login to view
思路分析
To determine the number of sigma and pi bonds in a molecule, we first analyze its Lewis structure or skeletal formula and count bonds by type.
- General rule: Each single bond consists of one sigma bond. Each multiple bond contains one sigma bond plus additional pi bonds equal to the bond order minus one. For example, a double bond has one sigma and one pi; a triple bond has one sigma......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
The Lewis structure for molecule below has _____ σ bonds and ______ π bonds.
Given the Lewis structure below: There are [Blank-1] sigma bonds and [Blank-2] pi bonds.
sigma bonds are stronger than pi bonds
How many sigma and pi bonds are in the molecule shown below? Sigma bonds: [ Select ] 3 6 8 2 9 4 5 7 0 1 10 Pi bonds: [ Select ] 9 5 7 10 3 6 4 2 0 8 1
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!