题目
CHEM1012 (L2) Online Quiz #1
多项填空题
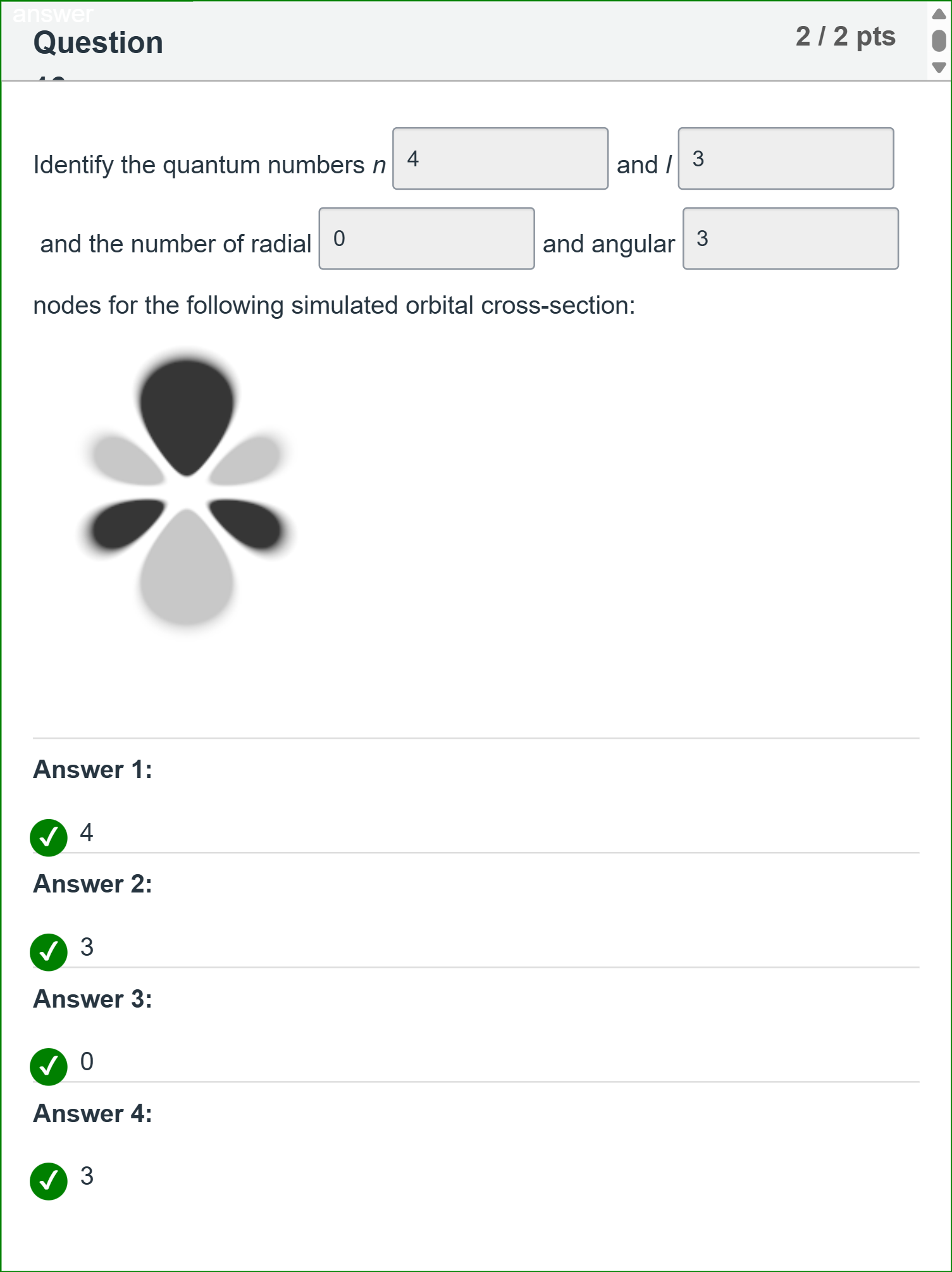
Identify the quantum numbers n [Fill in the blank] and l [Fill in the blank] and the number of radial [Fill in the blank] and angular [Fill in the blank] nodes for the following simulated orbital cross-section:
查看解析
标准答案
Please login to view
思路分析
To unpack the problem, we are asked to identify four quantities for a given simulated orbital cross-section: the principal quantum number n, the azimuthal (orbital) quantum number l, the number of radial nodes, and the number of angular nodes.
Option 1: n = 4. The principal quantum number n must be greater than the azimuthal quantum number l (n......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
There are [Fill in the blank] orbitals in a 5p subshell. The angular momentum quantum number (l) of these orbitals is [Fill in the blank] . (Input numbers only.)
Identify the quantum numbers n [Fill in the blank] and l [Fill in the blank] and the number of radial [Fill in the blank] and angular [Fill in the blank] nodes for the following simulated orbital cross-section:
Orbitals with the same principal quantum number (n) are said to be in the same shell.
Which quantum number must be the same for the electrons in the three orbitals shown below? 17A
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!