题目
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 16 Quiz C07-4-6
多项选择题
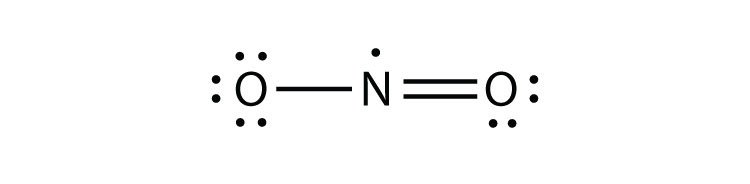
What category of octet exception does this molecule belong to? [Select all that apply.]
选项
A.Molecules or polyatomic ions with an odd number of electrons
B.Molecules or polyatomic ions with atoms that have fewer than an octet of valence electrons
C.Molecules or polyatomic ions with atoms that have more than an octet of valence electrons
D.This molecule does not violate the octet rule
查看解析
标准答案
Please login to view
思路分析
First, consider the electron count of the molecule shown, O=N=O. The total valence electrons come from O (6) + N (5) + O (6) = 17 electrons, which is an odd number.
Option 1: Molecules or polyatomic ions with an odd number of electrons. This is correct because an odd total number of valence electrons leads to a radical species, meaning the octet rule is not satisfied for a......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
What category of octet exception does this molecule belong to?
Which atoms are likely to form stable molecules that have an incomplete octet on the central atom? [Select all that apply.]
This question and the one that follows will ask questions about the three structures drawn in this video: I3-, POCl3, and BF3. Draw all three structures and match them to the reason they are allowed to break the octet rule. (hint: you can use the same answer more than once) 1: I_3^-1 2: POCl_3 3: BF_3
In a consumer society, many adults channel creativity into buying things
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!