题目
CHM1052 - MUM S2 2025 Week 6: Preparation quiz
单项选择题
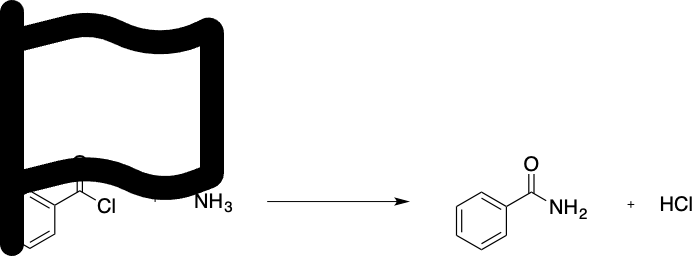
Consider the nucleophilic addition of ammonia to benzoyl chloride shown below. Which of the following statements is true?
选项
A.a. No reaction occurs because the amide product is less stable than benzoyl chloride
B.b. Chloride anions are poor leaving groups because they are not stable in solution
C.c. Chloride anions are good leaving groups because they are stable in solution
D.d. Ammonia is the electrophile
E.e. Benzoyl chloride is the nucleophile
查看解析
标准答案
Please login to view
思路分析
The question asks about the nucleophilic addition to benzoyl chloride with ammonia and which statement is true.
Option a: 'No reaction occurs because the amide product is less stable than benzoyl chloride.' This is not accurate. Amide formation from acyl chlorides by amines is a common, favorable reaction driven by formation of a stable amide and by the good leaving group chloride, so claiming no react......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Consider the nucleophilic substitution of ammonia to benzoyl chloride shown below. Which of the following statements is true?
Compound (I) reacts with methanol to produce intermediate (II) and chloride ions. Which one of the following mechanisms is correct?
Consider the reaction of ethanoyl chloride with methanol shown below. What product(s) may you expect to be formed from the reaction?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!