题目
CHM1052 - MUM S2 2025 CHM1052 2022 practice exam 1
单项选择题
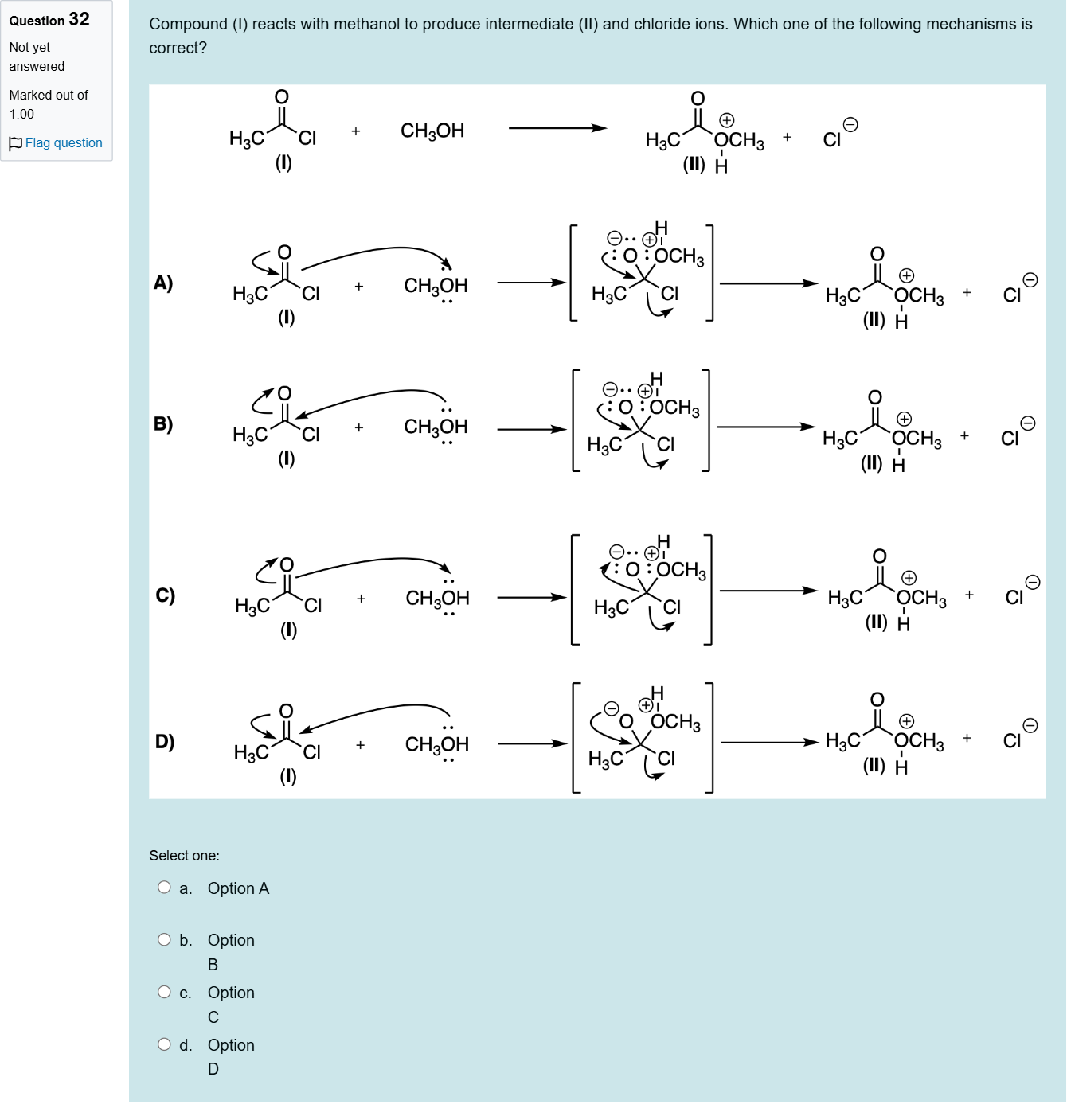
Compound (I) reacts with methanol to produce intermediate (II) and chloride ions. Which one of the following mechanisms is correct?
选项
A.a. Option A
B.b. Option B
C.c. Option C
D.d. Option D
查看解析
标准答案
Please login to view
思路分析
This question asks you to evaluate which mechanistic sequence best describes the reaction of compound I with methanol to give intermediate II and chloride ions.
First, consider what is happening: a carbonyl chloride (acetyl chloride-type) plus methanol can proceed via nucleophilic attack by alcohol on the acyl carbon, forming a tetrahedral intermediate, followed by collapse to give an ester (as intermediate II) and release of chloride as Cl−. You should look for a mechanism where methanol acts as a nucleophile attacking the carbonyl carbon, the leaving group is Cl−, and the intermediate resembles a protonated or neutral ester formation prior ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Consider the nucleophilic substitution of ammonia to benzoyl chloride shown below. Which of the following statements is true?
Consider the reaction of ethanoyl chloride with methanol shown below. What product(s) may you expect to be formed from the reaction?
Consider the nucleophilic addition of ammonia to benzoyl chloride shown below. Which of the following statements is true?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!