题目
My LMS Subjects Quiz 4
数值题
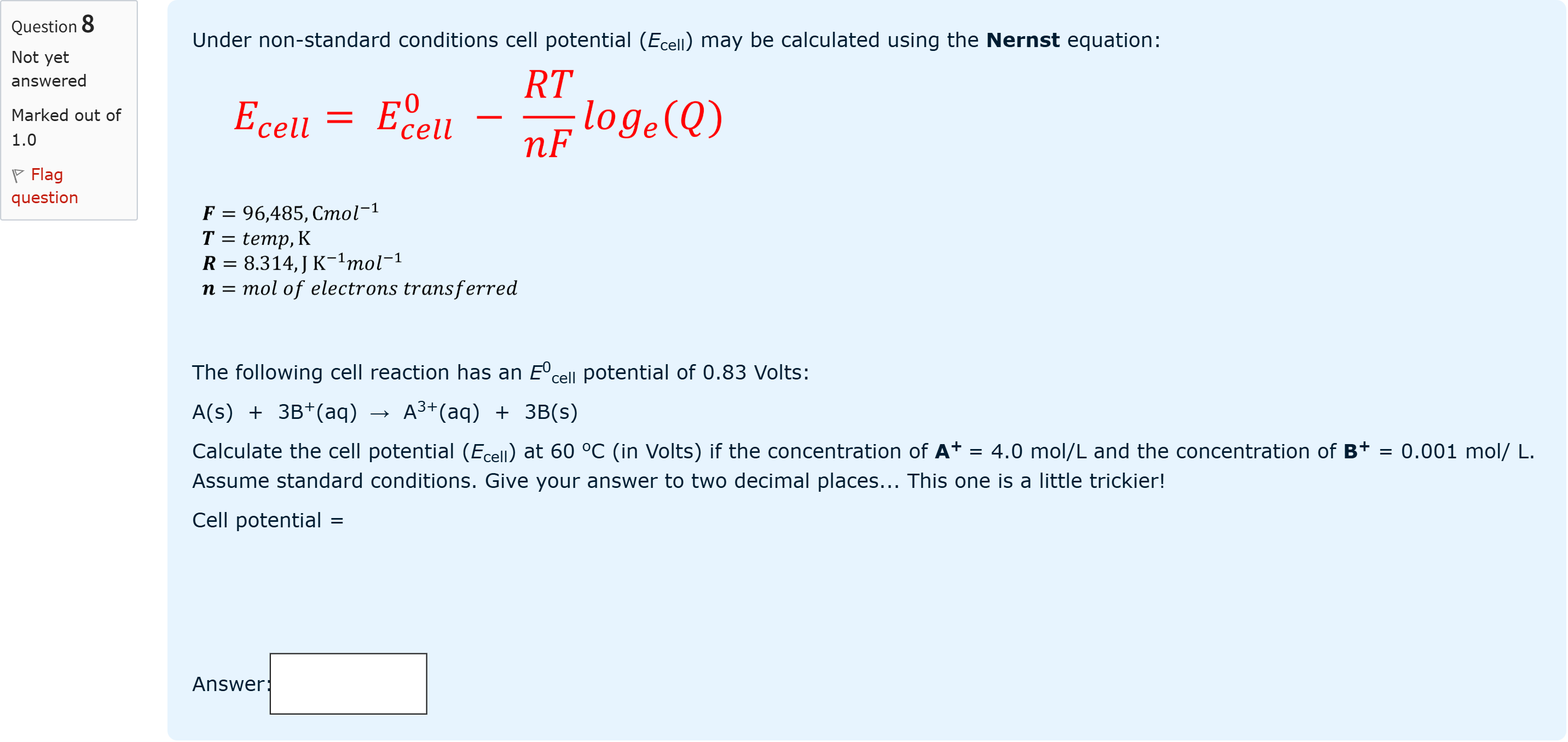
Under non-standard conditions cell potential (Ecell) may be calculated using the Nernst equation: The following cell reaction has an E0cell potential of 0.83 Volts: A(s) + 3B+(aq) → A3+(aq) + 3B(s) Calculate the cell potential (Ecell) at 60 oC (in Volts) if the concentration of A+ = 4.0 mol/L and the concentration of B+ = 0.001 mol/ L. Assume standard conditions. Give your answer to two decimal places... This one is a little trickier! Cell potential =
查看解析
标准答案
Please login to view
思路分析
To tackle this problem, we start from the Nernst equation for the cell reaction given: Ecell = E0cell − (RT/nF) ln Q.
The reaction is: A(s) + 3 B+(aq) → A3+(aq) + 3 B(s).
- n, the number of electrons transferred, equals 3 because the A goes from A(s) to A3+(aq) (A3+ gains 3 electrons).
- E0cell is given as 0.83 V at standard conditions.
......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Under non-standard conditions cell potential (Ecell) may be calculated using the Nernst equation: The following cell reaction has an E0cell potential of 0.83 Volts: A(s) + B+(aq) → A+(aq) + B(s) Calculate the cell potential (Ecell) at 298 K (in Volts) if the concentration of A+ = 0.050 mol/L and the concentration of B+ = 5.0 mol/ L. Assume standard conditions. Give your answer to two decimal places. Do not include units. Cell potential =
The equilibrium constant of the reaction shown below is 2.41 × 108 at 25°C: 3Ag(s) + NO3—(aq) +4H+(aq) → 3Ag+(aq) + NO(g) + 2H2O(l) Calculate the value of E°cell for a cell utilizing this reaction.
Consider the following voltaic cell: Ni(s)Ni2+(aq)Cl2(g)Cl–(aq)Pt(s) If the standard potentials of the Ni2+/Ni and Cl2/Cl– couples are –0.23 V and +1.36 V, respectively, at 25 °C calculate the voltage of this cell when {Ni2+] = 0.100 M, [Cl–] = 0.100 M, and the pressure of Cl2(g) = 1.50 atm.
Consider the following voltaic cell: Ni(s)∣Ni2+(aq)∥Cl2(g)∣Cl–(aq)∣Pt(s) If the standard potentials of the Ni2+/Ni and Cl2/Cl– couples are –0.23 V and +1.36 V, respectively, at 25 °C calculate the voltage of this cell when {Ni2+] = 0.100 M, [Cl–] = 0.100 M, and the pressure of Cl2(g) = 1.50 atm.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!