题目
CHM1051 MUM S2 2025 CHM1051 Practice Exam 1
单项选择题
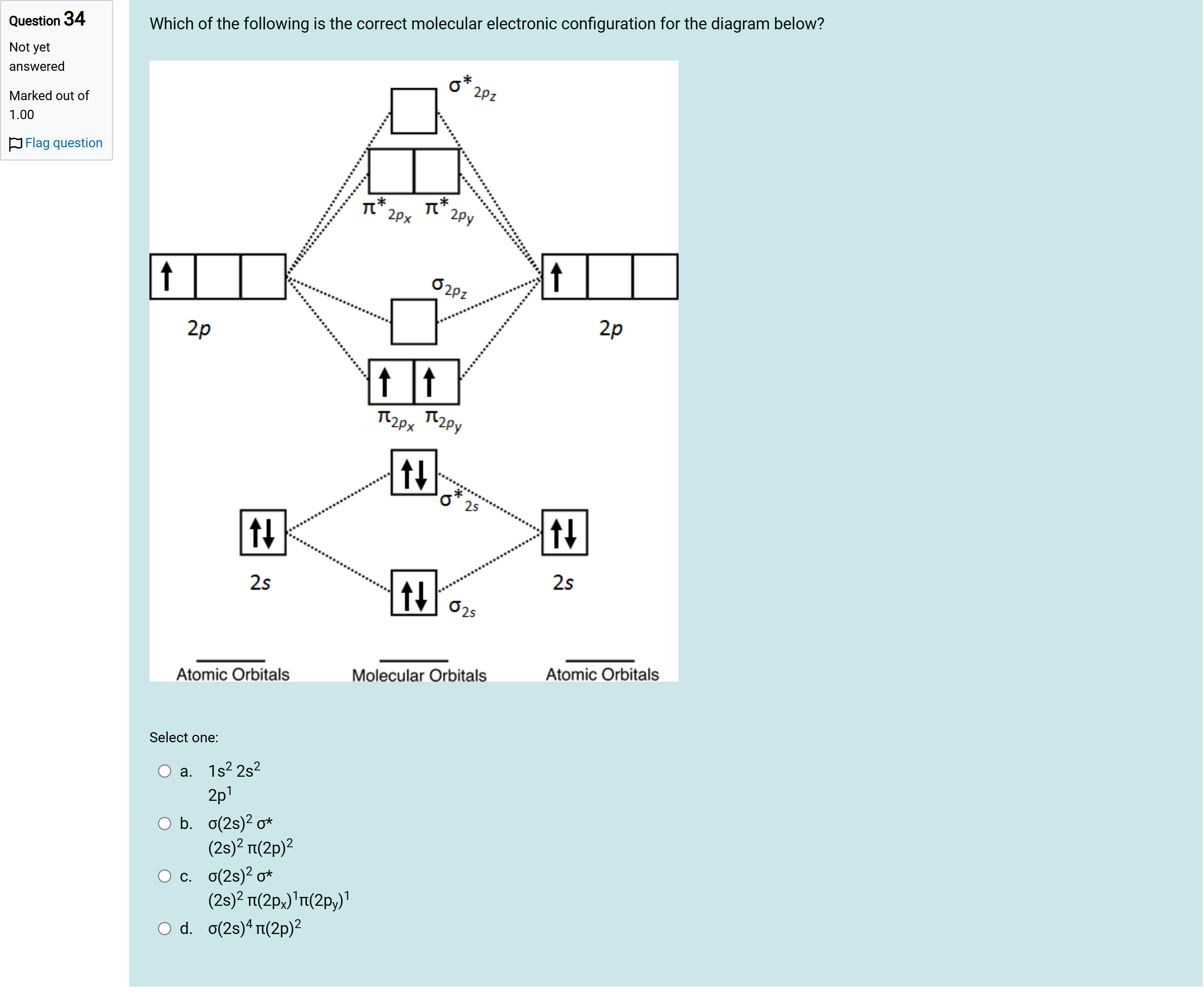
Which of the following is the correct molecular electronic configuration for the diagram below?
选项
A.a. 1s2 2s2 2p1
B.b. σ(2s)2 σ*(2s)2 π(2p)2
C.c. σ(2s)2 σ*(2s)2 π(2px)1π(2py)1
D.d. σ(2s)4 π(2p)2
查看解析
标准答案
Please login to view
思路分析
First, I will restate the question and the available choices to set the context for analysis.
Question: Which of the following is the correct molecular electronic configuration for the diagram below?
Options:
- a. 1s2 2s2 2p1
- b. σ(2s)2 σ*(2s)2 π(2p)2
- c. σ(2s)2 σ*(2s)2 π(2px)1π(2py)1
- d. σ(2s)4 π(2p)2
Now, let’s examine each option in relation to the diagram provided (which shows electrons in σ(2s), σ*(2s), and the two degenerate π orbitals π(2px) and π(2py)).
Option a: 1s2 2s2 2p1
- This ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Using the molecular orbital theory, predict how many unpaired electrons are in the B22– ion.
Using molecular orbital theory, predict the bond order for the O2+ ion.
Assuming an MO diagram with orbital energies like that of O2 what is the bond order and number of unpaired electrons for the molecule NO2- ?
Using the molecular orbital theory, predict how many unpaired electrons are in the O2+ ion.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!