你还在为考试焦头烂额?找我们就对了!
我们知道现在是考试月,你正在为了考试复习到焦头烂额。为了让更多留学生在备考与学习季更轻松,我们决定将Gold会员限时免费开放至2025年12月31日!原价£29.99每月,如今登录即享!无门槛领取。
助你高效冲刺备考!
题目
CHEM 1210 AU2025 (15738) CHEM 1210 Practice Exam #4 v2 (Ch 7.1–7.4, 8, 9.1–9.6)- Requires Respondus LockDown Browser
单项选择题
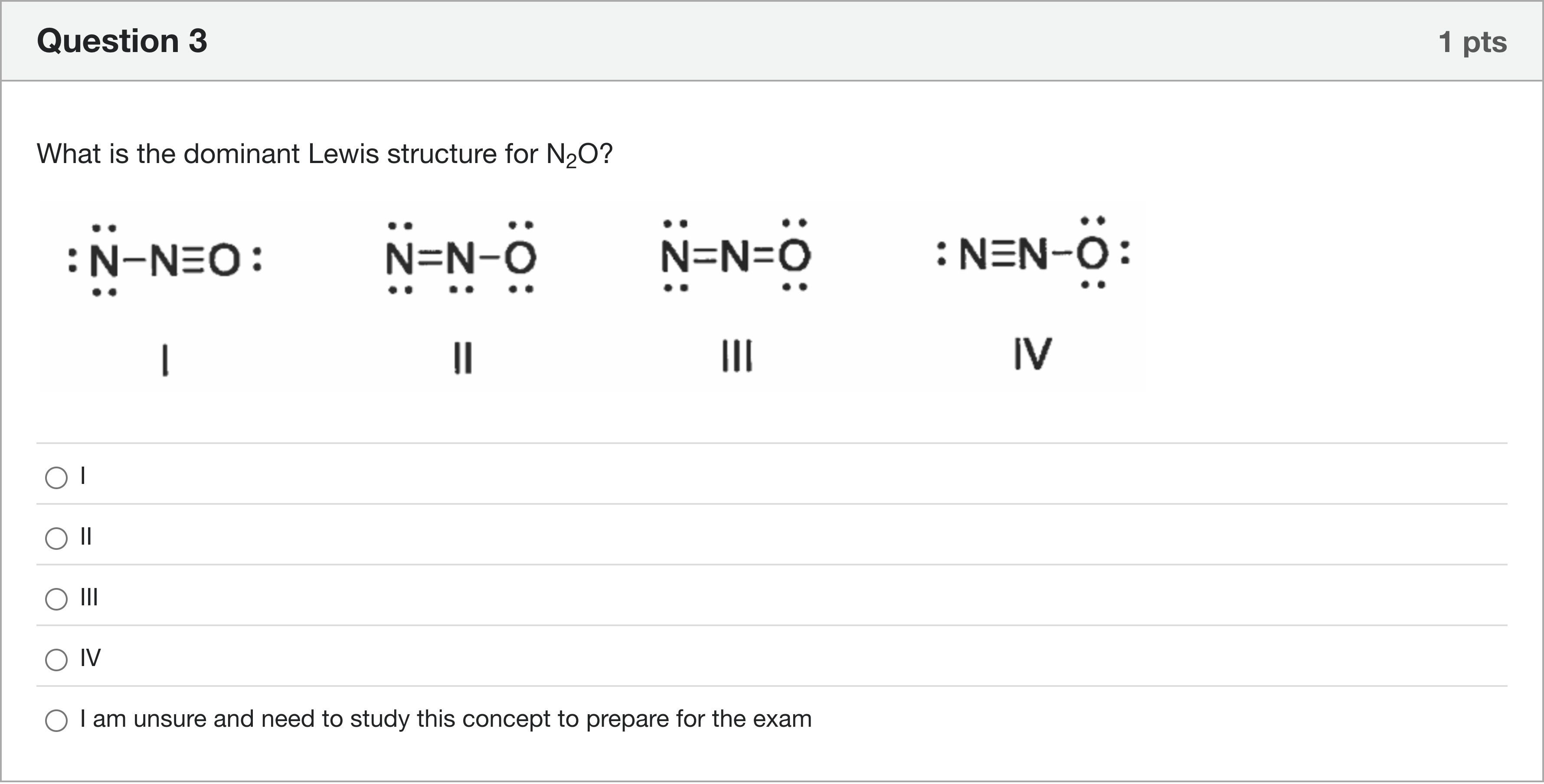
What is the dominant Lewis structure for N2O?
选项
A.I
B.II
C.III
D.IV
E.I am unsure and need to study this concept to prepare for the exam
查看解析
标准答案
Please login to view
思路分析
Question: What is the dominant Lewis structure for N2O?
Options: I, II, III, IV, I am unsure and need to study this concept to prepare for the exam
Step-by-step analysis of each option:
Option I: This structure likely places bonds and lone pairs in a way that either violates octet rules for one of the atoms or assigns an unfavorable distribution of formal charges. If any atom ends up with an incomplete octet or an excessive formal charge on a less electronegative atom, this form is less favorable as a canonical contributor.
Rationale: For N2O, the dominant form should satisfy the octet for N and O as much as possible and place the negative formal charge on the more electroneg......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following Lewis structures are incorrect? There may be more than one.
In the most plausible Lewis structure for SOF4, there are
Draw the most stable Lewis structure for H2CS and complete the following statements. I: The total number of nonbonding pairs of electrons on the sulfur atom is [ Select ] 0 3 1 2 . II: The bond between the carbon and sulfur atoms is a [ Select ] triple bond double bond single bond . 07A
Based on formal charges, which of the three Lewis structures shown for CH2ON– is the dominant Lewis structure? 10A
更多留学生实用工具
希望你的学习变得更简单
为了让更多留学生在备考与学习季更轻松,我们决定将Gold 会员限时免费开放至2025年12月31日!