你还在为考试焦头烂额?找我们就对了!
我们知道现在是考试月,你正在为了考试复习到焦头烂额。为了让更多留学生在备考与学习季更轻松,我们决定将Gold会员限时免费开放至2025年12月31日!原价£29.99每月,如今登录即享!无门槛领取。
助你高效冲刺备考!
题目
CHEM1011 (ND) Lecture quiz: Week 6
单项选择题
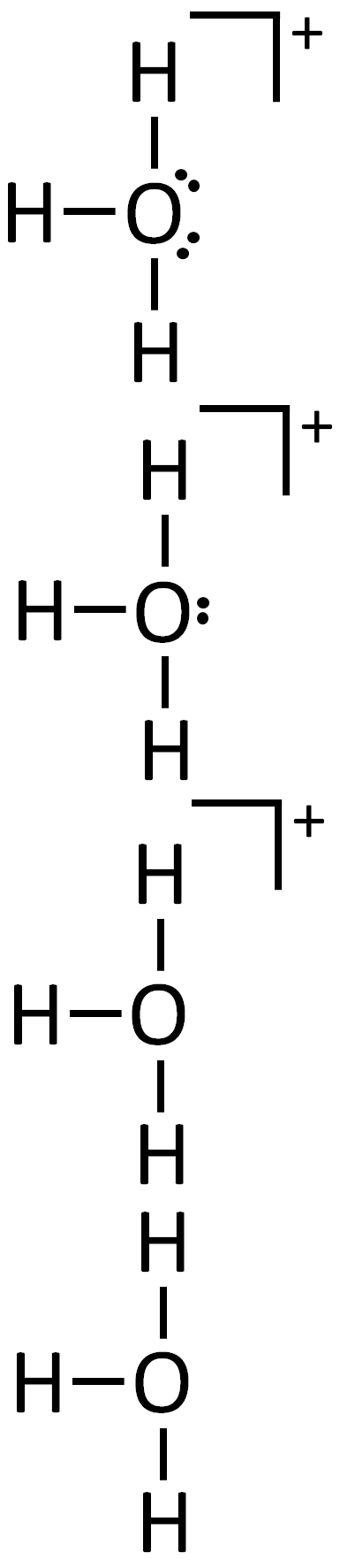
Identify the correct Lewis structure of H3O+. Ensure that all charges and lone pairs are shown.
选项
A.
B.
C.
D.
查看解析
标准答案
Please login to view
思路分析
Question restatement: Identify the correct Lewis structure of H3O+ and ensure charges and lone pairs are shown.
Since the answer options provided are blank, I will reason generally about the correct Lewis structure and common incorrect possibilities, so you can recognize the right structure if you see it.
Key facts to apply:
- Hydronium ion H3O+ consists of an oxygen atom covalently bonded to three hydrogen atoms.
- Oxygen brings 6 valence electrons; each O–H bond uses 2 electrons, and there are three such bonds, totaling 6 electrons used in bonding.
- The overall spec......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following Lewis structures are incorrect? There may be more than one.
In the most plausible Lewis structure for SOF4, there are
Draw the most stable Lewis structure for H2CS and complete the following statements. I: The total number of nonbonding pairs of electrons on the sulfur atom is [ Select ] 0 3 1 2 . II: The bond between the carbon and sulfur atoms is a [ Select ] triple bond double bond single bond . 07A
Based on formal charges, which of the three Lewis structures shown for CH2ON– is the dominant Lewis structure? 10A
更多留学生实用工具
希望你的学习变得更简单
为了让更多留学生在备考与学习季更轻松,我们决定将Gold 会员限时免费开放至2025年12月31日!