题目
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 23 Quiz C10-2-3
多重下拉选择题
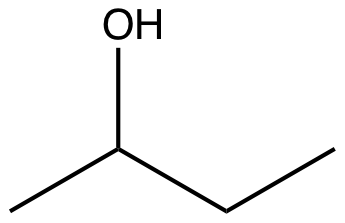
How many carbon atoms, hydrogen atoms, and lone pairs are in the molecule shown below? Carbons: 4 Hydrogens: 10 Lone Pairs: 2
查看解析
标准答案
Please login to view
思路分析
To analyze this question properly, I’ll treat it as a three-part count: the number of carbon atoms, the number of hydrogen atoms, and the number of lone pairs in the depicted molecule.
First, consider the carbon count. If the provided answer indicates 4 carbons, we would verify by tracing the molecular skeleton: count each distinct carbon atom present in the structure, including those in rings or chains, and excluding any duplicates due to resonance or symmetry if applicable. The figure’s labeling (Carbons: 4) aligns with a small hydrocarbon framework typical of a four-carbon backbone.
Next, the hydrogen count. The given value is 10 hydrogens. To validate this, one would......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following Lewis structures are incorrect? There may be more than one.
In the most plausible Lewis structure for SOF4, there are
Draw the most stable Lewis structure for H2CS and complete the following statements. I: The total number of nonbonding pairs of electrons on the sulfur atom is [ Select ] 0 3 1 2 . II: The bond between the carbon and sulfur atoms is a [ Select ] triple bond double bond single bond . 07A
Based on formal charges, which of the three Lewis structures shown for CH2ON– is the dominant Lewis structure? 10A
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!