你还在为考试焦头烂额?找我们就对了!
我们知道现在是考试月,你正在为了考试复习到焦头烂额。为了让更多留学生在备考与学习季更轻松,我们决定将Gold会员限时免费开放至2025年12月31日!原价£29.99每月,如今登录即享!无门槛领取。
助你高效冲刺备考!
题目
单项选择题
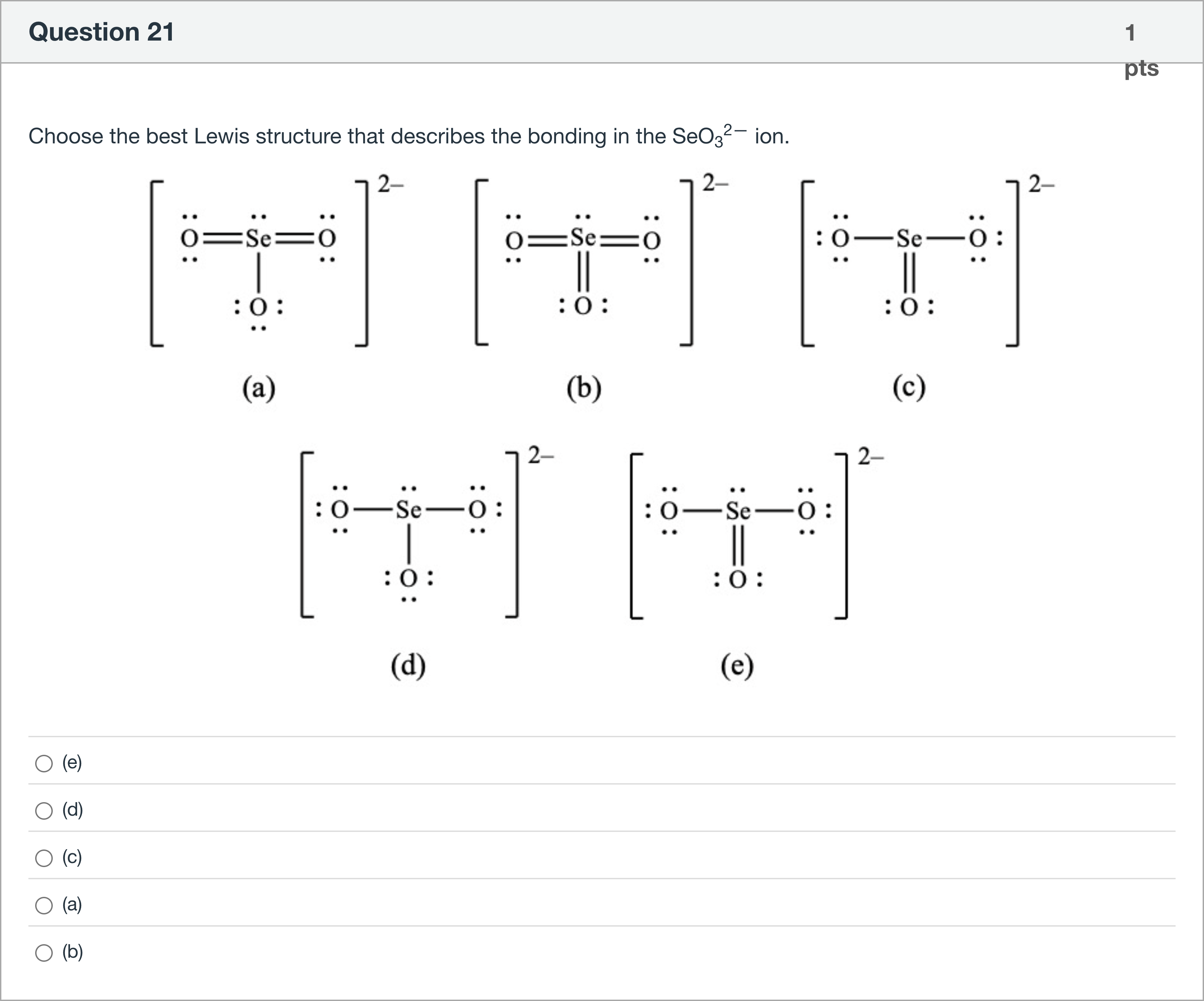
Choose the best Lewis structure that describes the bonding in the SeO32— ion.
选项
A.(e)
B.(d)
C.(c)
D.(a)
E.(b)
查看解析
标准答案
Please login to view
思路分析
Question restatement: Choose the best Lewis structure that describes the bonding in the SeO3^{2−} ion.
Option-by-option analysis:
- Option (a): This structure shows Se forming three bonds to oxygen atoms with two double bonds and one single bond, or some arrangement that leaves Se without a lone pair. If Se has no lone pair in this depiction, its formal charge distribution would not properly sum to the overall 2− charge, and the octet rule would be violated for Se in a way that contradicts known chemistry for Se in the +6 oxidation state with expanded octets. The lack of a clearly placed lone pair on Se also tends to misrepresent the formal charges on the oxygen atoms.
- Option (b): In this drawing, Se appears to be bonded to three oxygens with one of the bonds be......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following Lewis structures are incorrect? There may be more than one.
In the most plausible Lewis structure for SOF4, there are
Draw the most stable Lewis structure for H2CS and complete the following statements. I: The total number of nonbonding pairs of electrons on the sulfur atom is [ Select ] 0 3 1 2 . II: The bond between the carbon and sulfur atoms is a [ Select ] triple bond double bond single bond . 07A
Based on formal charges, which of the three Lewis structures shown for CH2ON– is the dominant Lewis structure? 10A
更多留学生实用工具
希望你的学习变得更简单
为了让更多留学生在备考与学习季更轻松,我们决定将Gold 会员限时免费开放至2025年12月31日!