题目
My LMS Subjects Quiz 3
数值题
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to one decimal point.
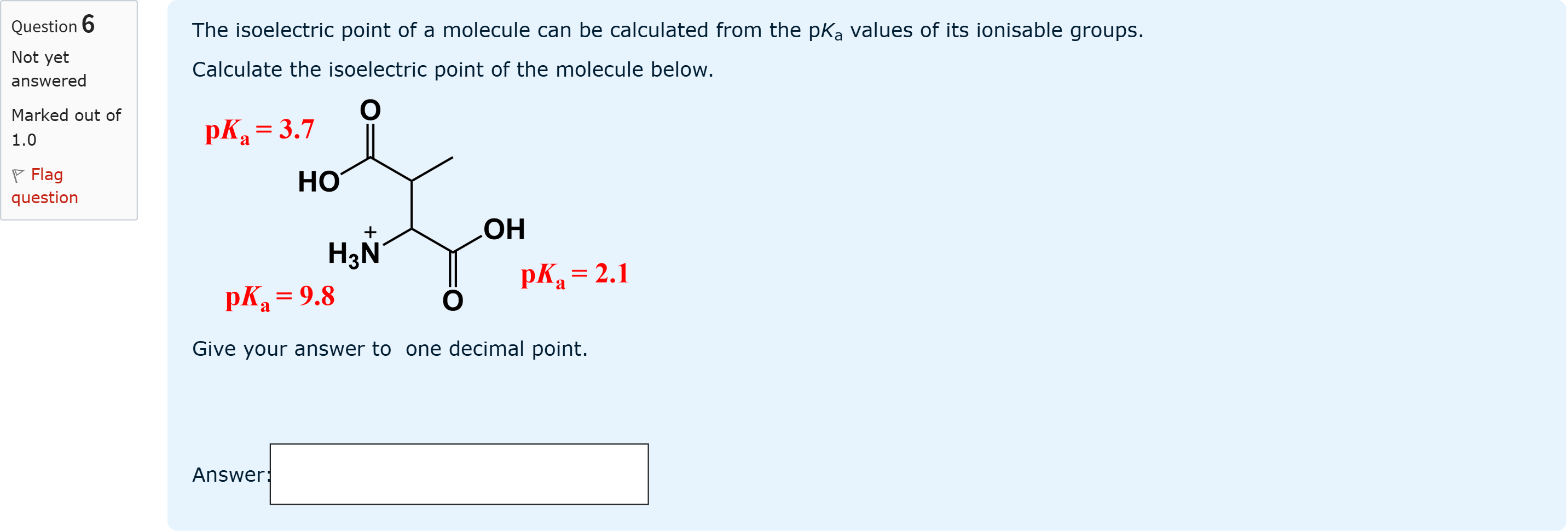
查看解析
标准答案
Please login to view
思路分析
To determine the isoelectric point (pI) for this molecule, we first identify the ionizable groups and their pKa values shown in the diagram. The molecule has two carboxyl groups (with pKa values 2.1 and 3.7) and one amine gr......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to two decimal points.
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups. Calculate the isoelectric point for the molecule below. Give your answer to one decimal point.
Which one of the following amino acids has the highest isoelectric point? (all amino acids are shown at the same pH)
The pI is the pH where a molecules charge is overall neutral . This is sometimes at near pH 7.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!