题目
AP Chemistry A Unit 1 quiz
单项选择题
The ionization energies for element X are listed in the table above. On the basis of the data, element X is most likely to be
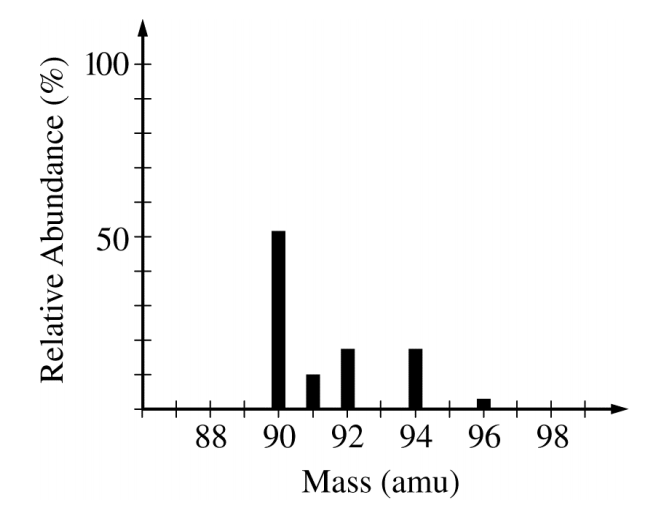
查看解析
标准答案
Please login to view
思路分析
The question asks us to identify element X based on its ionization energy data. Since the answer options are not provided in the prompt, I’ll focus on explaining why Aluminum (Al) is consistent with a characteristic ionization-energy pattern and how that pattern differs from nearby elements.
- Understanding ionization energy patterns: In atoms, the first ionization energy (IE1) is the energy required to remove the first electron from a neutral atom. For elements in a given group, the number of valence electrons often shows up as a notable change (a large jump) after removing all valence electrons. For Grou......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Given the following elements and three values of possible first ionization energies: Si, Na, Cl and 496, 1251, and 786 kJ·mol–1 Match the atoms with their ionization energies.
Nonmetals rarely lose electrons in chemical reactions because
For the elements Be, B, N and O, the order of increasing ionization energies are:
Which of the following equations represents the second ionization energy of calcium? 04A
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!