题目
CHEM 1210 AU2025 (15738) CHEM 1210 Practice Exam #4 v2 (Ch 7.1–7.4, 8, 9.1–9.6)- Requires Respondus LockDown Browser
单项选择题
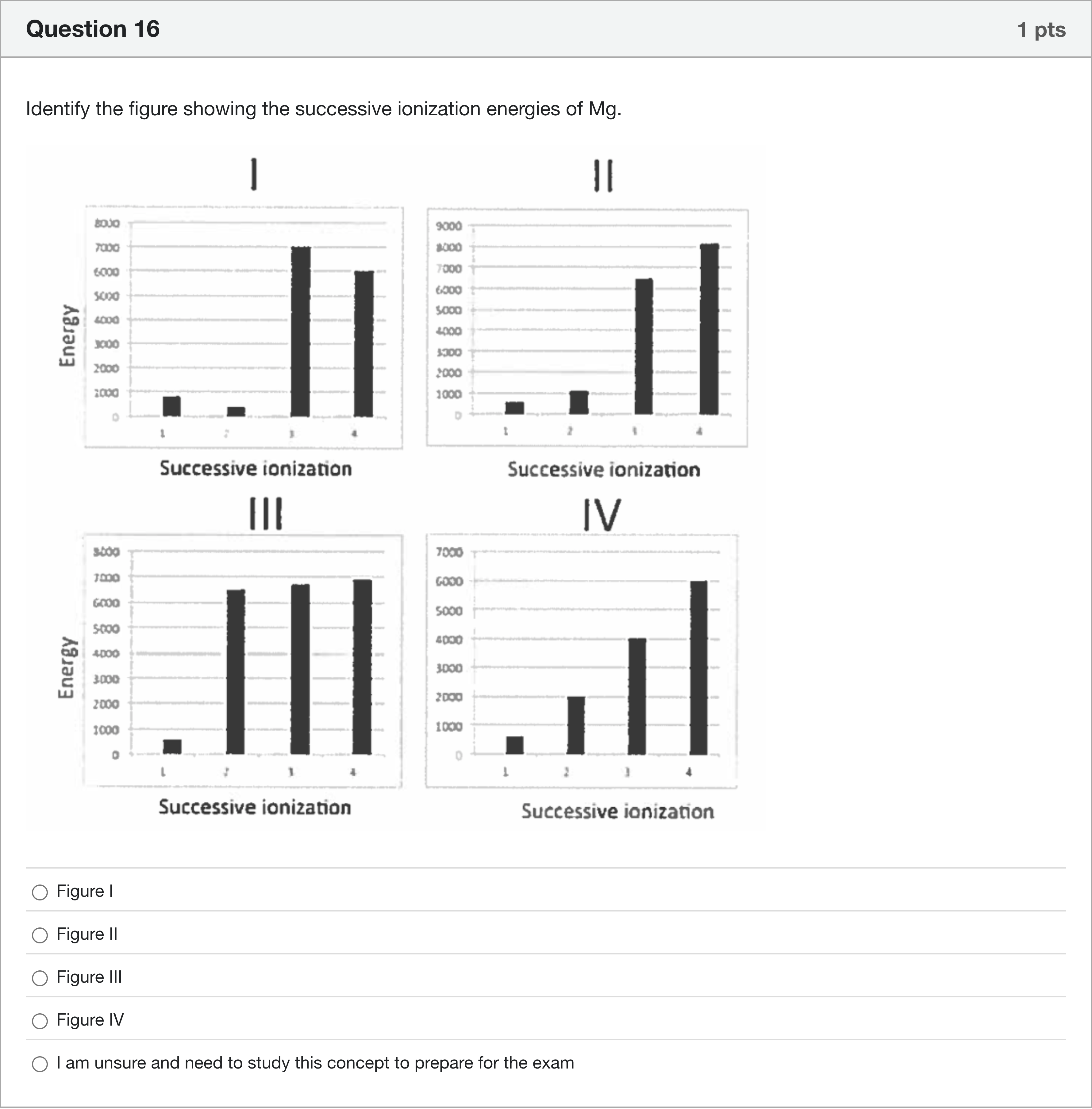
Identify the figure showing the successive ionization energies of Mg.
选项
A.Figure I
B.Figure II
C.Figure III
D.Figure IV
E.I am unsure and need to study this concept to prepare for the exam
查看解析
标准答案
Please login to view
思路分析
To identify the figure showing Mg’s successive ionization energies, I will compare the general pattern of ionization energies for a metal with a noble-gas core like magnesium.
- Concept to apply: Magnesium has the electron configuration [Ne] 3s^2. The first two ionizations remove the two 3s electrons, which require relatively small amounts of energy. Once those two valence electrons are removed, the next electron is taken from a closed-shell (Ne) core, which requires a substantially larger amount of energy. Consequently, there is a dramatic jump in ionization energy after the second ionizat......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Given the following elements and three values of possible first ionization energies: Si, Na, Cl and 496, 1251, and 786 kJ·mol–1 Match the atoms with their ionization energies.
Nonmetals rarely lose electrons in chemical reactions because
For the elements Be, B, N and O, the order of increasing ionization energies are:
Which of the following equations represents the second ionization energy of calcium? 04A
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!