题目
单项选择题
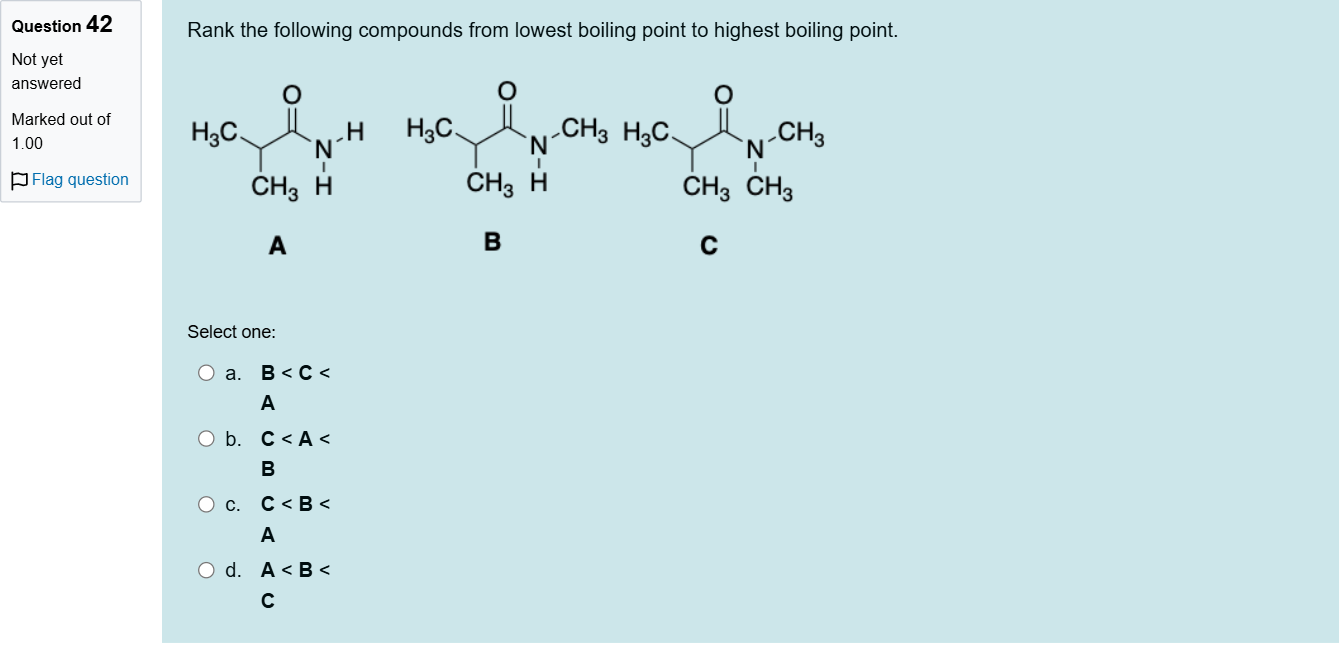
Rank the following compounds from lowest boiling point to highest boiling point.
选项
A.a. B < C < A
B.b. C < A < B
C.c. C < B < A
D.d. A < B < C
查看解析
标准答案
Please login to view
思路分析
Question restatement: Rank the following compounds from lowest boiling point to highest boiling point. The answer field indicates the ranking: C < B < A. Answer options: a. B < C < A; b. C < A < B; c. C < B < A; d. A < B < C.
To compare boiling points, consider the key factors that influence BP in organic compounds: intermolecular hydrogen bonding, molecular weight, and molecular structure/branching which affects surface area and dispersion forces.
Option a (B < C < A): If this were the correct ranking, it would imply B has the lowest BP, followed by C, with A having the highest. We must examine whether B truly would have a lower BP than C. If B can form stronger hydrogen bonding or has greater molecular weig......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Choose which of the following statements explains why benzoic acid is soluble in water.[Fill in the blank]
Question at position 34 Select all of the weak types of bonds.IonicHydrogenVan der WaalsCovalent
Match the following properties of liquids with their best definition 1: Surface tension ____ 2: Cohesive force ____ 3: Capillary action ____ 4: Adhesive forces ____
What type of intermolecular forces are due to the attraction between temporary dipoles and their induced temporary dipoles?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!