题目
单项选择题
Which of the following is constant for 1 mole of any ideal gas?
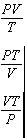
查看解析
标准答案
Please login to view
思路分析
The question asks which quantity is constant for 1 mole of any ideal gas.
First, recall the ideal gas law: PV = nRT.
Since the question specifies 1 mole, set n = 1, giving PV = RT.
From this, you can derive severa......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Which of the following statement is correct regarding to the equation, 𝑃 𝑉 = 𝑛 𝑅 𝑇
Consider a sample of gas in a tank. Under which of the following conditions would you expect the most ideal behaviour?
Ideal gas law is an equation of state that relates the pressure, volume and temperature for a given mass of an ideal gas.
Ideal gas law is an equation of state that relates the pressure, volume and temperature for a given mass of an ideal gas.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!