题目
CHM1052 - MUM S2 2025 CHM1052 2022 practice exam 1
单项选择题
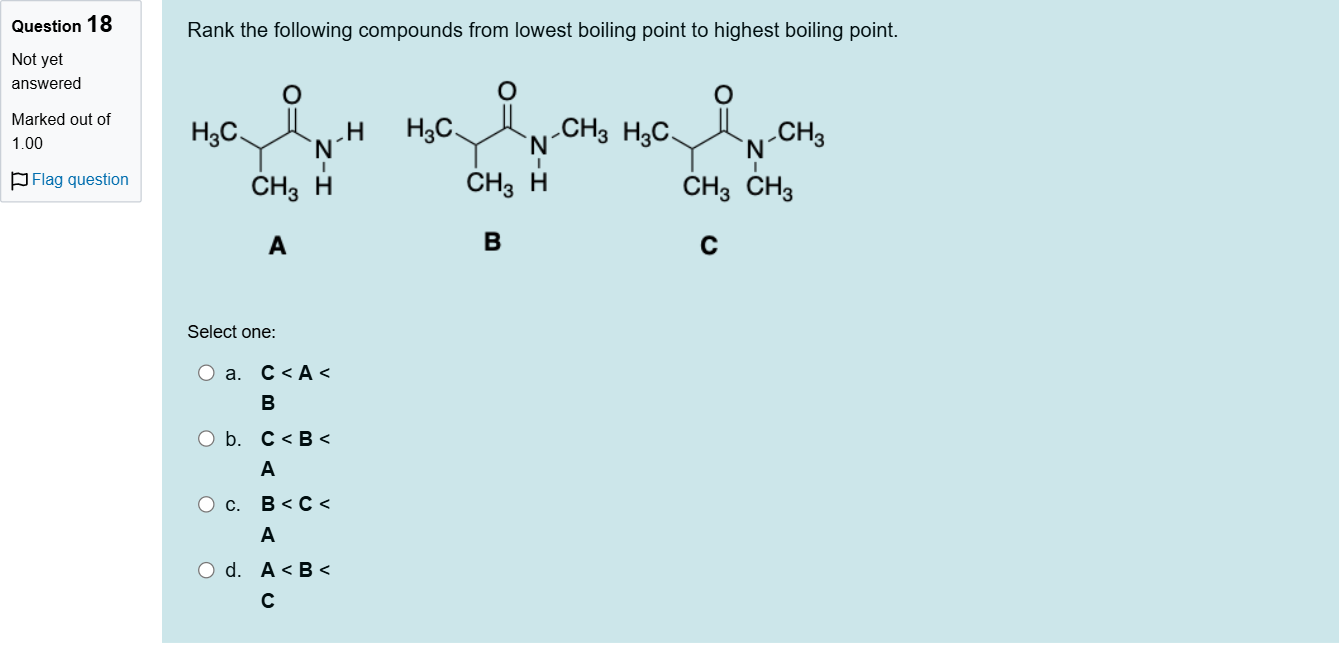
Rank the following compounds from lowest boiling point to highest boiling point.
选项
A.a. C < A < B
B.b. C < B < A
C.c. B < C < A
D.d. A < B < C
查看解析
标准答案
Please login to view
思路分析
When ranking boiling points among compounds of similar molecular weight, several factors come into play: hydrogen bonding capability, molecular polarity, and molecular symmetry/branching which affects surface area.
Option a: 'C < A < B' would mean A has a higher boiling point than B. If A possesses more accessible hydrogen-bond donors/acceptors or a larger, less-branched surface area than B, this could push A’s bp above B. However, given typical amide-like structures with substantial hydrogen-bonding networks, a large, highly ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Question at position 87 When two water molecules interact with each other, the partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called?a hydrophobic bonda covalent bonda hydrophilic bondan ionic bonda hydrogen bond
Hydrogen bonds form between the hydrogen atoms of water molecules and
Question at position 14 What type of bond do 2 water molecules form with each other?hydrogen bondVan der Waals bondcovalent bondpolar covalent bondionic bond
For the three amide compounds shown below, choose the order which represents the amide compound that has the highest boiling point to the one with the lowest boiling point.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!