题目
MUF0121 Physics Unit 1 - Semester 2, 2025
数值题
54 kJ of heat was absorbed by a system, whilst also having 33 kJ of work done by it. What is the change in the internal energy of this system after these processes?
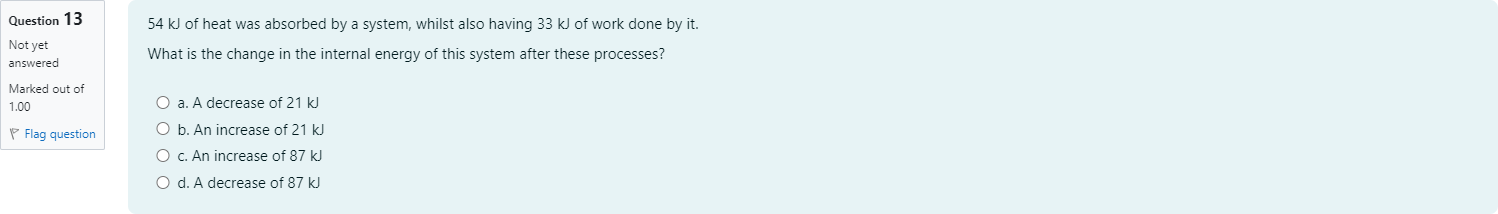
查看解析
标准答案
Please login to view
思路分析
The problem describes a thermodynamics scenario where a system absorbs heat and does work on its surroundings. According to the first law of thermodyna......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
The surroundings do 120 J of work on a gas. At the same time, the gas releases 23 J of heat. What is ΔE for this process? 02A
A perfectly insulated system has work done by it at a rate of 20 W. At what rate is the internal energy of the system changing?
A perfectly insulated system has work done by it at a rate of 13 W. At what rate is the internal energy of the system changing?
A system has a heat source supplying heat at a rate of 187 W and is doing work at a rate of 130 W. At what rate is the internal energy of the system changing?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!