题目
AGRI10046_2025_SM2 Week 4 progress quiz
单项选择题
Consider the reaction below: 3C(s) + 3H2(g) ⥫⥬ CH4(g) + C2H2(g) The equilibrium expression for the reaction above is:
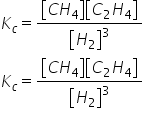
查看解析
标准答案
Please login to view
思路分析
The question asks for the equilibrium expression for the given reaction. Since no answer options are provided, I’ll first lay out the general form of the expression and explain the role of each species.
Step 1: Write the balanced equation as given: 3 C(s) + 3 H2(g) ⇌ CH4(g) + C2H2(g).
Step 2: Apply the equilibrium expression. For a reaction aA +......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Use the data given to calculate the value of Κ for the reaction at 5°C Ag+(aq) + Cl− (aq) AgCl(s) AgCl(s) Ag+(aq) Cl− (aq) S° (J K−1 mol−1) 96.2 72.68 56.4 ΔH°f (kJ/mol) −127.07 105.58 −167.2
Use the data given to calculate the value of Κ for the reaction at 25°C AgCl(s) Ag+(aq) + Cl− (aq) AgCl(s) Ag+(aq) Cl− (aq) S° (J K−1 mol−1) +96.2 +72.68 +56.4 ΔH°f (kJ/mol) −127.07 +105.58 −167.2
The equilibrium constant expression for the reaction shown below is Ag3PO4(s) 3 Ag+(aq) + PO43− (aq)
Express the equilibrium constant for the following reaction. 10 PCl5(g) 10 PCl3(g) + 10 Cl2(g)
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!