题目
My LMS Subjects Practice LMS questions, Week 3
单项选择题
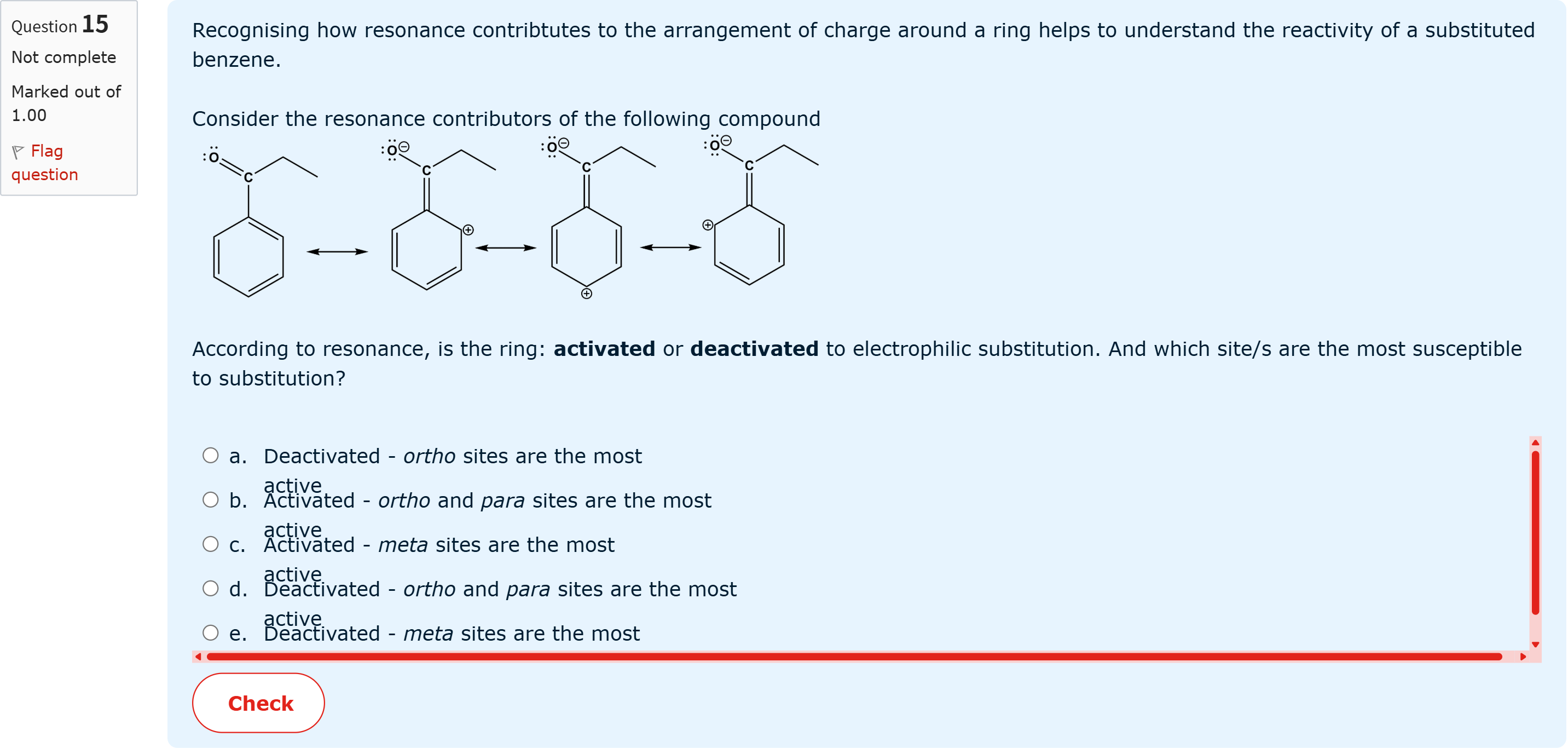
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
选项
A.a. Deactivated - ortho sites are the most active
B.b. Activated - ortho and para sites are the most active
C.c. Activated - meta sites are the most active
D.d. Deactivated - ortho and para sites are the most active
E.e. Deactivated - meta sites are the most active
查看解析
标准答案
Please login to view
思路分析
The question asks us to evaluate resonance effects on a substituted benzene ring and determine both the overall reactivity (activated or deactivated) toward electrophilic substitution and which positions are most susceptible.
Option a: Deactivated - ortho sites are the most active. This is inconsistent with common directing effects: when a ring is deactivated, it is usually due to electron-withdrawing groups, and the ortho/para positions are not ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
Which of the following, will provide a meta, nitro (NO2) compound, upon nitration with HNO3/H2SO4?
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!