题目
单项选择题
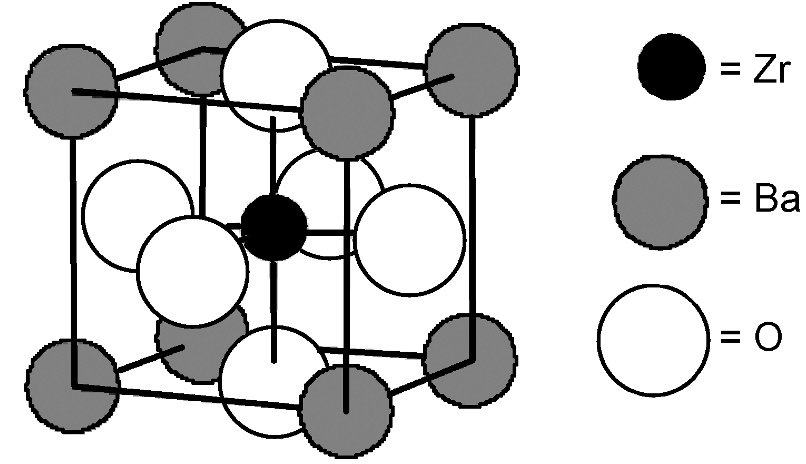
An ionic solid composed of Ba, Zr, and O ions has the cubic unit cell shown above. The unit cell has barium atoms at the corners, a zirconium atom in the cube center, and oxygen anions in the cube faces. What is the empirical formula of the compound?
选项
A.B
a
Z
r
O
6
B.B
a
Z
r
O
4
C.B
a
8
Z
r
O
3
D.None of the others.
E.B
a
8
Z
r
O
6
查看解析
标准答案
Please login to view
思路分析
The question asks for the empirical formula of an ionic solid with Ba at the corners of a cubic unit cell, Zr at the cube center, and O on the faces.
First, determine the contributions: Ba at corners contribute 1 Ba per formula unit (8 corners × 1/8 each = 1). Zr at the center contributes 1 Zr per formula unit (1 atom per cell). O at the faces contribute 3 O per formula ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Question at position 11 Which statements below are true? Select all that apply.All minerals are crystalline (have a regular arrangement of atoms).During mineral growth, crystals can form if sufficient space and sufficient time are available.All other things being equal, covalently-bonded minerals are harder (have stronger bonds) than those with van der Waals bonds.Elements with filled outermost electron shells are chemically inert (non-reactive).
What does it mean to be crystalline? Choose the best answer.
The smallest group of particles in a crystal that retains the shape of the crystal is called the ____.
Which one oft he following has a structure, which is very different to the other three?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!