题目
My LMS Subjects Practice LMS quiz, Week 1
单项选择题
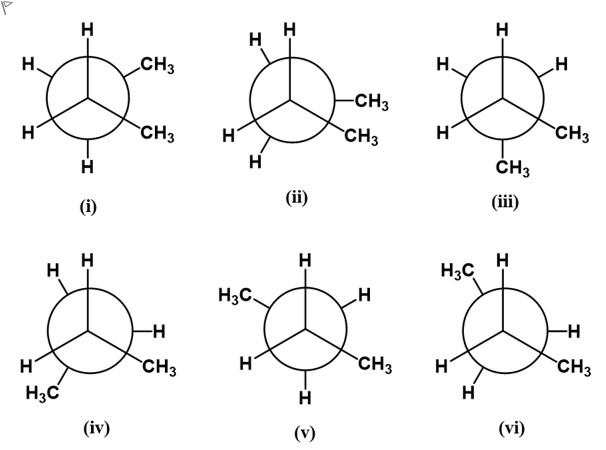
Consider the Newman projections (i - vi) looking down the C2 - C3 bond in n-butane. Select the highest energy conformer of n-butane?
选项
A.a. i
B.b. ii
C.c. iii
D.d. iv
E.e. v
F.f. vi
查看解析
标准答案
Please login to view
思路分析
To determine the highest-energy conformer for n-butane when looking down the C2–C3 bond, we compare the interactions between substituents on the two adjacent carbons in each Newman projection. The key idea is that eclipsing interactions are higher in energy than staggered ones, and among eclipsed forms, the eclipse of two methyl groups (CH3–CH3) is the most energetic due to steric crowding.
Option a (i): Analyze whether in this projection the two front and back substituents are eclipsing or staggered. If the two methyl groups are not directly eclipsing each other and the conformer i......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
When considering molecular conformations, which of the following statements are true?
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O6 (i – v) in the chair conformation.Which of i – v will be the lowest energy (most stable)?
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O1Br5 (i – v) in the chair conformation.Which of i – v will be the highest energy (least stable)?
Consider the following Newman projections (i – iii) of an alkylhalide showing all the staggered conformations about the C2-C1 bond. Key: X = a bulky halide group.Which of the following lists the conformers, i – iii, from lowest to highest energy?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!