题目
My LMS Subjects Quiz 3
单项选择题
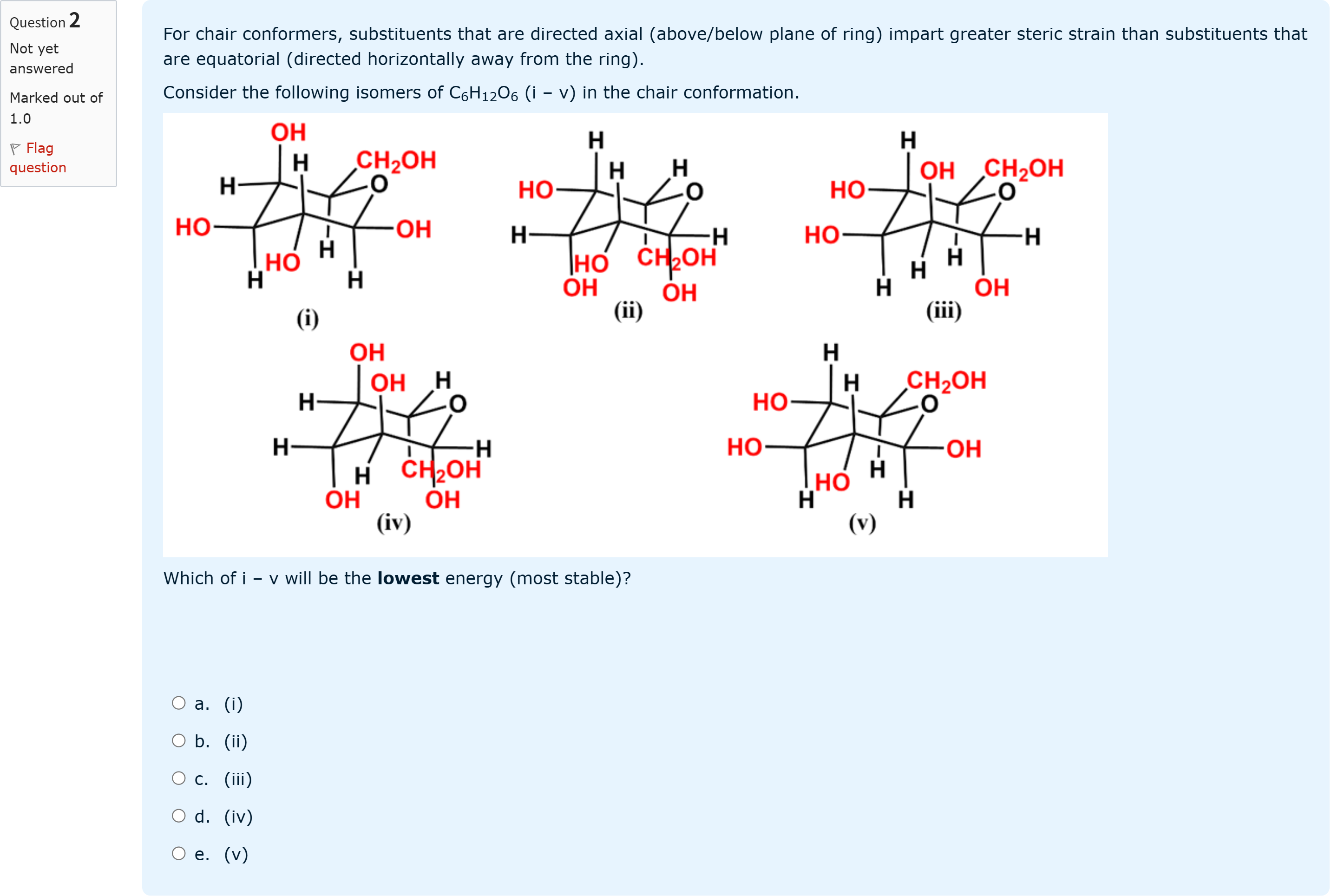
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O6 (i – v) in the chair conformation.Which of i – v will be the lowest energy (most stable)?
选项
A.a. (i)
B.b. (ii)
C.c. (iii)
D.d. (iv)
E.e. (v)
查看解析
标准答案
Please login to view
思路分析
The question asks which chair conformer among i–v will have the lowest energy, i.e., be the most stable, given that axial substituents cause more steric strain than equatorial ones.
Option a: (i). In structure (i), several substituents appear axial (the red OH groups on both sides may be axial positions depending on the chair flip). If many substituents occupy axial sites, steric strain from 1,3-diaxial interactions would be high, making this conf......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
When considering molecular conformations, which of the following statements are true?
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O1Br5 (i – v) in the chair conformation.Which of i – v will be the highest energy (least stable)?
Consider the following Newman projections (i – iii) of an alkylhalide showing all the staggered conformations about the C2-C1 bond. Key: X = a bulky halide group.Which of the following lists the conformers, i – iii, from lowest to highest energy?
Consider the Newman projections (i - vi) looking down the C2 - C3 bond in n-butane. Select the LOWEST energy conformer of n-butane?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!