题目
单项选择题
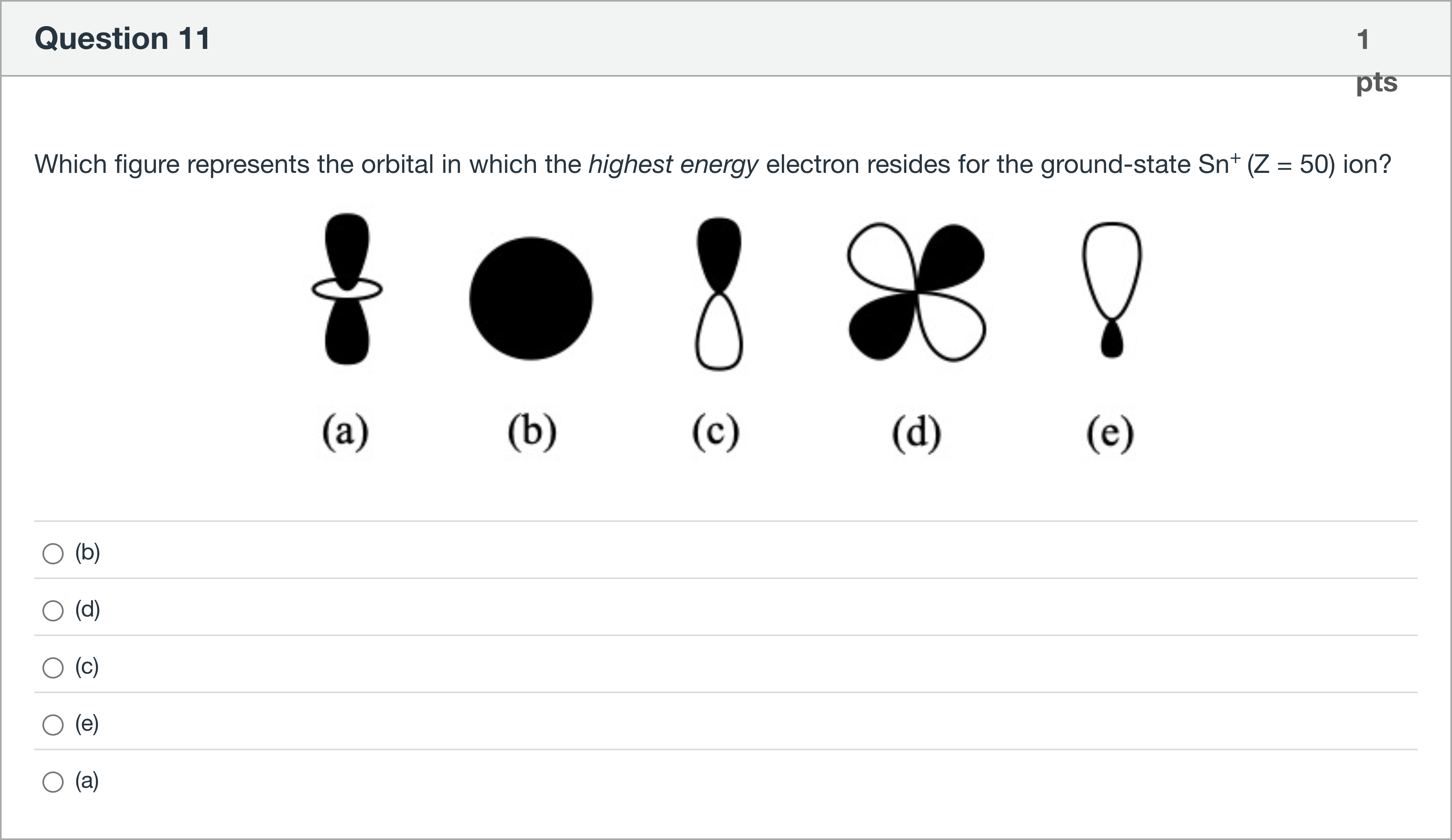
Which figure represents the orbital in which the highest energy electron resides for the ground-state Sn+ (Z = 50) ion?
选项
A.(b)
B.(d)
C.(c)
D.(e)
E.(a)
查看解析
标准答案
Please login to view
思路分析
Question restated: Which figure represents the orbital in which the highest energy electron resides for the ground-state Sn+ (Z = 50) ion?
First, identify the electronic configuration of Sn in the ground state and for Sn+. Tin (Sn) has atomic number 50. Its neutral electron configuration ends with 5p2, specifically [Kr] 4d10 5s2 5p2. When it loses one electron to form Sn+ (Z = 50, +1 charge), the removed electron comes from the highest occupied subshell, which is the 5p orbital. Therefore, the highest energy electron in Sn+ resides in a 5p orbital, i.e., a p-type orbital.
Next, evaluate the shapes of the options to map them to orbital types:
- Option (b): The diagram shows a solid sphere, which......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Consider the following orbital in the n=5 shell: This orbital has [Fill in the blank] angular node(s) and [Fill in the blank] radial node(s). (Input numbers only.)
Orbitals with larger principal quantum number (n) have a smaller size.
The atomic orbital for an electron in an atom with the quantum numbers n = 4, l = 3, ml = 0, ms = – 1 2 is
Which of the period 4 orbitals below is a 3dyz orbital?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!