题目
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 6 Quiz C02-1-3
单项选择题
The arrows in the figure depict the transition of an electron in the H atom. Which transition requires the absorption of the highest energy photon?
选项
A.n = 1 to n = 3
B.n = 2 to n = 6
C.n = 3 to n = 2
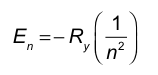
查看解析
标准答案
Please login to view
思路分析
To determine which transition requires the absorption of the highest energy photon, we compare the energy gaps between the initial and final levels for each upward (absorption) transition.
Option A: n = 1 to n = 3.......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
The hydrogen emission spectrum only shows a few discrete emission lines. This is because:
When an atom emits a photon, how does the photon's energy depend on the energy of the electron orbits?
Which one of the following is the energy of an electron in the n = 5 level of a hydrogen atom?
The lowest energy of an electron in atom is called?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!