题目
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 6 Quiz C02-1-3
多项选择题
The arrows in the figure depict the transition of an electron in the H atom. A photon is absorbed when the transition occurs. Which transition requires the absorption of the highest energy photon?
选项
A.n = 1 to n = 2
B.n = 2 to n = 4
C.n = 3 to n = 7
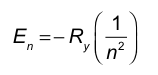
查看解析
标准答案
Please login to view
思路分析
To compare the energy of the absorbed photon, use the hydrogen atom energy levels En = -R/n^2. For an absorption transition from ni to nf (nf > ni), the photon energy is ΔE = Ef - Ei = [-R/(nf^2)] - [-R/(ni^2)] = R(1/ni^2 - 1/nf^2).
Option 1: n =......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
The hydrogen emission spectrum only shows a few discrete emission lines. This is because:
When an atom emits a photon, how does the photon's energy depend on the energy of the electron orbits?
Which one of the following is the energy of an electron in the n = 5 level of a hydrogen atom?
The lowest energy of an electron in atom is called?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!