题目
单项选择题
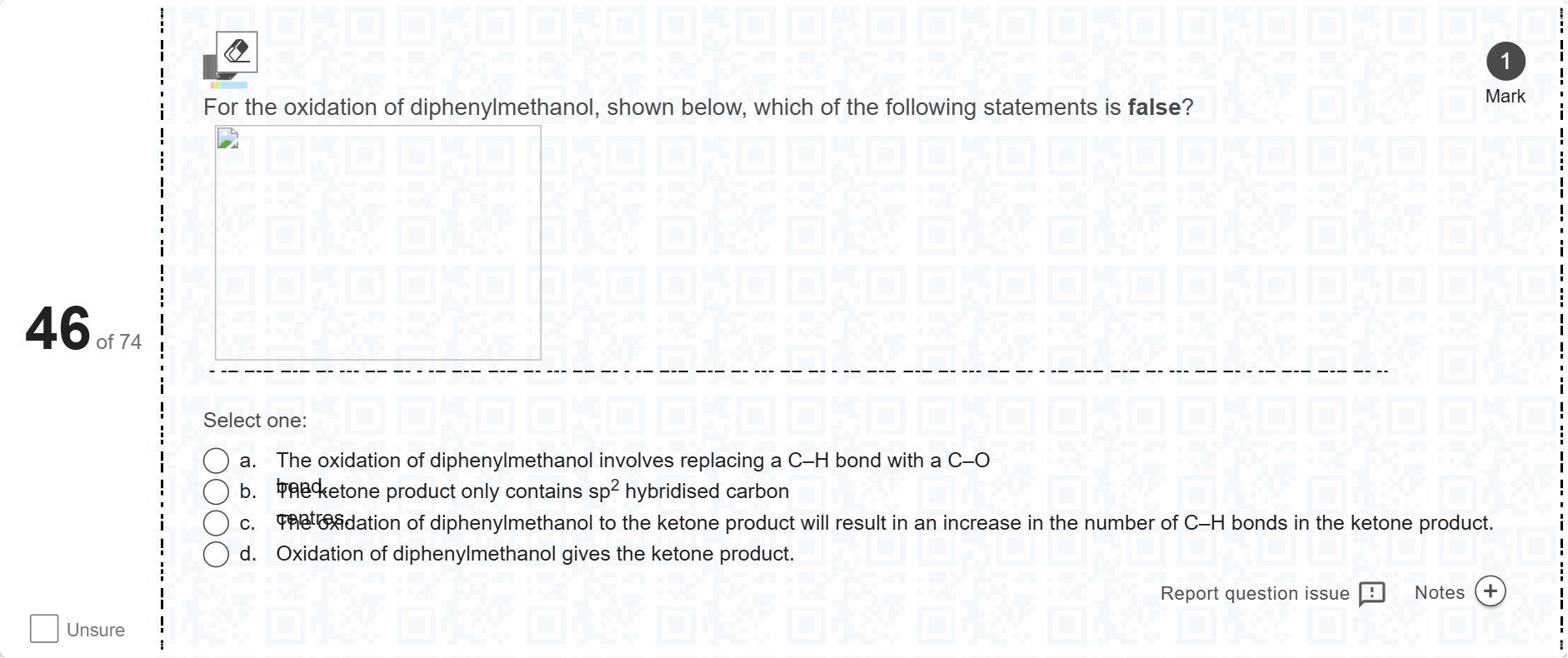
For the oxidation of diphenylmethanol, shown below, which of the following statements is false?[Fill in the blank]
选项
A.a. The oxidation of diphenylmethanol involves replacing a C–H bond with a C–O bond.
B.b. The ketone product only contains sp2 hybridised carbon centres.
C.c. The oxidation of diphenylmethanol to the ketone product will result in an increase in the number of C–H bonds in the ketone product.
D.d. Oxidation of diphenylmethanol gives the ketone product.
查看解析
标准答案
Please login to view
思路分析
To tackle this question, I will examine each provided statement about the oxidation of diphenylmethanol to the corresponding ketone, addressing what is true or false based on standard oxidation chemistry and structural implications.
Option a: 'The oxidation of diphenylmethanol involves replacing a C–H bond with a C–O bond.' In an oxidation of a secondary alcohol to a ketone, the carbon bearing the hydroxyl group loses the hydrogen (and the oxygen forms a carbonyl). Conceptually......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Select True or False for the following statements: False Oxidation of a primary alcohol always gives an aldehyde. False H2SO4, HNO3, and H2CrO4 are all common oxidizing agents for alcohols. False Many organic reactions involve oxidizing an alkane to an alcohol directly. False PCC can oxidize primary, secondary, and tertiary alcohols to C=O.
Compound A reacts with both PCC and H2CrO4 to give the same oxygen containing product. Compound A is:
The oxidation of a secondary alcohol with acidified dichromate will produce
Which one of the of the following would not react with an acidified solution of potassium dichromate?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!