题目
MCD4400 Chemistry II - Trimester 3 - 2025
单项选择题
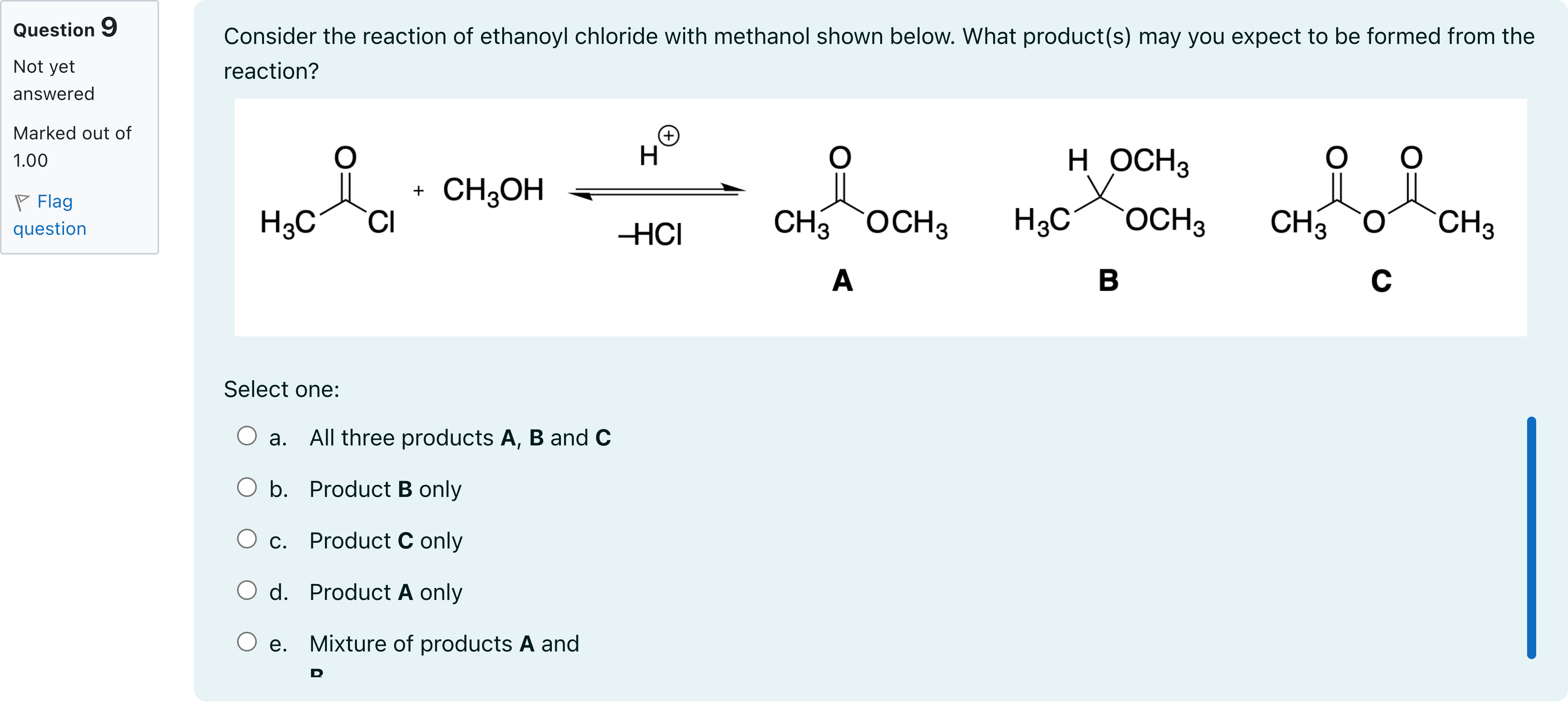
Consider the reaction of ethanoyl chloride with methanol shown below. What product(s) may you expect to be formed from the reaction?
选项
A.a. All three products A, B and C
B.b. Product B only
C.c. Product C only
D.d. Product A only
E.e. Mixture of products A and B
查看解析
标准答案
Please login to view
思路分析
First, consider the reaction type: ethanoyl chloride (CH3COCl) reacting with methanol (CH3OH) in the presence of an acid catalyst. This is a typical acyl substitution where the chloride leaving group is replaced by an alkoxy group, yielding an ester and releasing HCl.
Option a: All three products A, B and C. This would require multiple competing reaction pathways leading to different ester or side products. In a straightforward acyl chloride + alcohol reaction, the sole......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
People born without creativity never can develop it
A product has a selling price of $20, a contribution margin ratio of 40% and fixed cost of $120,000. To make a profit of $30,000. The number of units that must be sold is: Type the number without $ and a comma. Eg: 20000
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!