题目
单项选择题
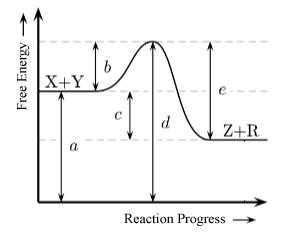
The energy diagram for the elementary reaction, X(g) + Y(g) ⇌ Z(g) + R(g) is shown below. What would happen if the kinetic energy of the reactants was not enough to provide the needed activation energy?
选项
A.The products would be produced at a lower energy state.
B.The reactants would not convert to products.
C.The products would form at an unstable energy state.
D.The activated complex would convert into products.
E.The rate of the reaction would tend to increase.
查看解析
标准答案
Please login to view
思路分析
To tackle this question, I will evaluate what happens when the kinetic energy of reactants is insufficient to overcome the activation energy barrier.
Option 1: "The products would be produced at a lower energy state." This statement misinterprets energy transfer in a reaction. Even if products could form, the energy diagram indicates that crossing the activation barrier is necessary; without enough energy, the system ......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
Question8 The activation energy of a reaction is: Select one alternative: the net change in free energy in an enzyme-catalysed system. negative if the reaction is energetically favourable. increased by enzymes. the energy that must be absorbed by reactants in order for the reaction to take place. lower for reactants than products. ResetMaximum marks: 1 Flag question undefined
In a one step reaction, the activation energy for the forward reaction is 20.0 kJ mol -1, and the enthalpy of the reaction is -30.0 kJ mol-1. Calculate the activation energy for the reverse reaction.
The rate constant of a reaction is measured at different temperatures. A plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of –8.55 103 K–1. What is the activation energy (Ea) for the reaction?
The rate constant of a reaction is measured at different temperatures. A plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of –8.55 × 103 K–1. What is the activation energy (Ea) for the reaction?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!