题目
CHM1052 - MUM S2 2025 Week 6: Preparation quiz
单项选择题
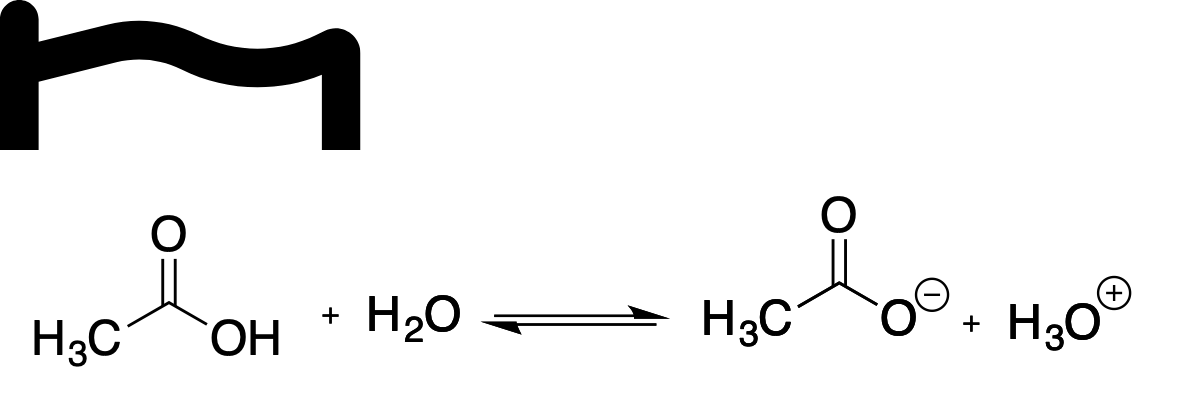
Which of the following statements correctly explains the dissociation of ethanoic acid in water?
选项
A.a. The equilibrium lies to the left hand side of the reaction equation
B.b. The carboxylate anion is resonance stabilised
C.c. Oxygen in an electropositive element
D.d. Water is acting as an electrophile
E.e. The generation of hydronium ions (H3O+) leads to an basic solution
查看解析
标准答案
Please login to view
思路分析
Let’s break down each statement in the context of the dissociation of ethanoic acid in water.
Option a: 'The equilibrium lies to the left hand side of the reaction equation.' This is the statement that the acid dissociation is not complete; ethanoic acid is a weak acid in water, so the undissociated form CH3COOH predominates and the equilibrium favors the left. This aligns with the known pKa ~4.76, meaning only a small fraction dissociates at typical conditions. Therefore, this option......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
In the reaction, HClO3 + N2H4 ⇌ ClO3– + N2H5+, which one of the sets below lists both of the acid species involved in the equilibrium?
If Kb for NH₃ is 1.8 × 10⁻⁵ at 25 0C, what is the pKa of its conjugate acid (NH₄⁺)?
In the reaction, HClO3 + N2H4 ⇌ ClO3– + N2H5+, which one of the sets below lists both of the acid species involved in the equilibrium?
An aqueous solution of acetic acid solution [CH3CO2H(aq)] has a pH of 3.25. Which of these substances raises the pH of the solution upon addition?
更多留学生实用工具
希望你的学习变得更简单
加入我们,立即解锁 海量真题 与 独家解析,让复习快人一步!