Questions
Multiple fill-in-the-blank
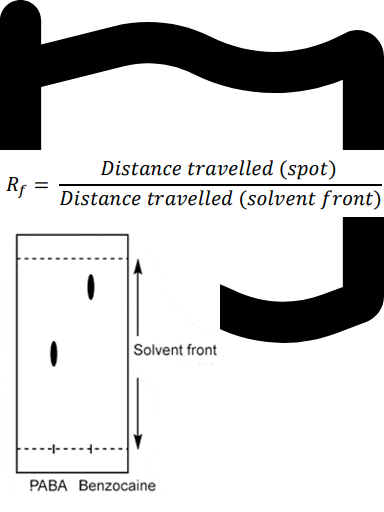
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Let’s break down the data given for the TLC results and then interpret what it means for polarity.
First, we calculate the Rf values using the formula: Rf = distance travelled by compound / distance travelled by solvent front.
For ρ-aminobenzoic acid (PABA): distance travelled = 2.1 cm, sol......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Question textAn example of Thin Layer Chromatography (TLC) results:Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate.Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures)Please refer to p.46 in the lab manual for more information.[table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cmBenzocaine: Distance travelled: 3.5 cmSolvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table]Based on the information above, which compound is less polar? Answer 3 Question 6[select: , Benzocaine, PABA, Neither]
Q6 V3Consider the following TLC plate of compounds X, Y and Z developed using a suitable mobile phase on a polar stationary phase. Which of the following is correct?
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Samples of three amino acids A, B and C, were spotted on the base line of a TLC plate. The chromatogram produced using a particular solvent is shown below: Amino acids Rf values alanine 0.56 isoleucine 0.79 taurine 0.34 Use the TLC plate and Rf values given to identify amino acid A.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!