Questions
MUF0042 Chemistry Unit 2 - Semester 2, 2025
Single choice
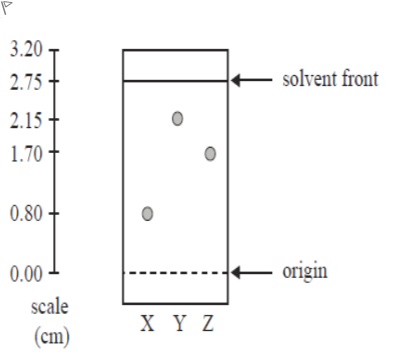
Q6 V3Consider the following TLC plate of compounds X, Y and Z developed using a suitable mobile phase on a polar stationary phase. Which of the following is correct?
Options
A.a. Z is more polar than X and has a higher Rf value than X
B.b. X is the most polar component and has an Rf value of 0.80
C.c. Z is more polar than Y and has a higher Rf value than Y
D.d. Y is the least polar component and has an Rf value of 0.78
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Question restatement: The TLC plate shows three components X, Y and Z developed on a polar stationary phase with a suitable mobile phase. We must choose which statement is correct about their relative polarity and Rf values.
Option a: 'Z is more polar than X and has a higher Rf value than X.' On a polar stationary phase, more polar compounds interact more strongly with the stationary phase, which lowers their Rf (they travel less). If Z were more polar than X, Z would typically have a lower Rf, n......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Question textAn example of Thin Layer Chromatography (TLC) results:Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate.Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures)Please refer to p.46 in the lab manual for more information.[table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cmBenzocaine: Distance travelled: 3.5 cmSolvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table]Based on the information above, which compound is less polar? Answer 3 Question 6[select: , Benzocaine, PABA, Neither]
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Question textAn example of Thin Layer Chromatography (TLC) results: Solvent used in developing a TLC plate: 50:50 hexane : ethyl acetate. Calculate the Retention factor (Rf) value for each component (ρ-aminobenzoic acid (PABA) and Benzocaine) on the plate. (to 2 significant figures) Please refer to p.47 in the lab manual for more information. [table] TLC results | Rf (retention factor) ρ-aminobenzoic acid (PABA):Distance travelled: 2.1 cm Benzocaine: Distance travelled: 3.5 cm Solvent front: Distance travelled: 4.1 cm | Rf (PABA)= Answer 1 Question 6 Rf (Benzocaine)= Answer 2 Question 6 [/table] Based on the information above, which compound is less polar? Multiple choice 1 Question 61. PABA2. Benzocaine3. NeitherMark 1.00 out of 1.00
Samples of three amino acids A, B and C, were spotted on the base line of a TLC plate. The chromatogram produced using a particular solvent is shown below: Amino acids Rf values alanine 0.56 isoleucine 0.79 taurine 0.34 Use the TLC plate and Rf values given to identify amino acid A.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!