Questions
PHAS0006_24-25 Quiz for week 1
Matching
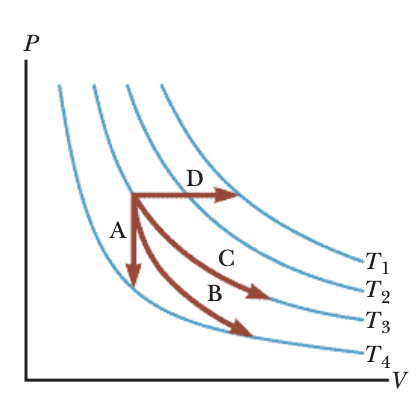
The image below shows a p-V diagram with isotherms indicated in blue. Match each of the marked paths A, B, C, D with the corresponding thermodynamic processes.
Options
A.Isobaric
B.Isothermal
C.Isosceles
D.Isovolumetric
E.Adiabatic
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Let's break down what each labeled path represents on a p–V diagram with the blue curves as isotherms.
A: The path A is drawn as a vertical segment, meaning the volume does not change while pressure changes. A vertical process on a p–V diagram corresponds to an isovolumetric (or isochoric) process. Since volume is fixed, heat transfer changes pressure, but volume remains constant. This matches the concept of isovolumetric.
B: The path B travels downward and to the right, moving to larger volume with decreasing pressure, along a traject......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Which choice correctly identifies the three processes shown in the diagrams?
In Stop to Think 19.2, which process results in the gas having a larger final temperature when the gas reaches State 3?
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!