Questions
Multiple fill-in-the-blank
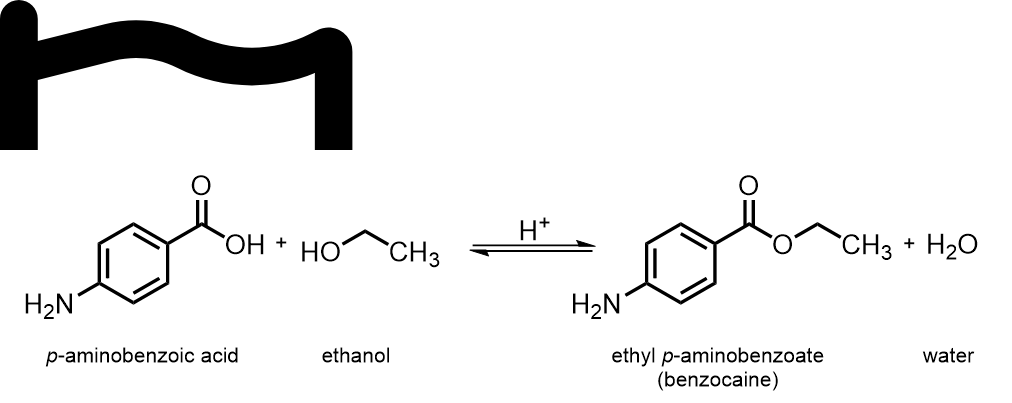
Question textBelow is the reaction you will be performing in the coming schedule week: To perform the synthesis you will be using the following reagents: [table] p-aminobenzoic acid (PABA) | ethanol M = 137.14 g mol-1 | M = 47.07 g mol-1 0.80 g | 10 mL (density: 0.789 g mL-1) [/table] Calculate (to 2 significant figures): No. of moles of PABA: Answer 1 Question 4[input] mol Mass of ethanol: Answer 2 Question 4[input] grams No. of moles of ethanol: Answer 3 Question 4[input] mol Determine the molar ratio of PABA : ethanol (round to the nearest integer): [table] PABA | : | ethanol Ratio = nPABA / nPABA | | Ratio = nethanol / nPABA Ratio = 1 | : | Answer 4 Question 4 [/table] Out of PABA and ethanol, which reagent will be used in excess? Multiple choice 1 Question 41. PABA2. Ethanol3. Neither is in excess – they will be used in their stoichiometric ratiosMark 1.00 out of 1.00
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
We start by identifying the given quantities and molar masses: PABA has M = 137.14 g/mol and mass = 0.80 g; ethanol has M = 47.07 g/mol (per the table) and volume = 10 mL with density 0.789 g/mL.
Step 1 — Number of moles of PABA: moles = mass / M = 0.80 g / 137.14 g/mol ≈ 0.00583 mol. Rounding to 2 significant figures gives 0.0058 mol.
Step ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Which of the following diagrams illustrates the law of multiple proportions?
If sodium hydrogen carbonate is added to a hydrochloric acid solution, it will produce gaseous carbon dioxide according to the following reaction: NaHCO3(s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g) How many grams of NaHCO3 must be added to an excess of HCl(aq) to produce 50.0 mL of CO2 at 25 °C and 0.995 atm?
Carbon monoxide and oxygen react to form carbon dioxide according to the following balanced chemical equation 2 CO(g) + O2(g) ➔ 2 CO2(g) If CO(g) and O2(g) are combined in the ratio shown in the diagram below, where the black spheres represent carbon atoms and the red spheres represent oxygen atoms, which molecules will remain when the reaction is complete?
Consider the following reaction: 4FeS2(s) + 11O2(g) → 2Fe2O3(s) + 8SO2(g) If 4.0 mol of FeS2 and 4.0 mol of O2 react, how many moles of Fe2O3(s) are produced?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!