Questions
Single choice
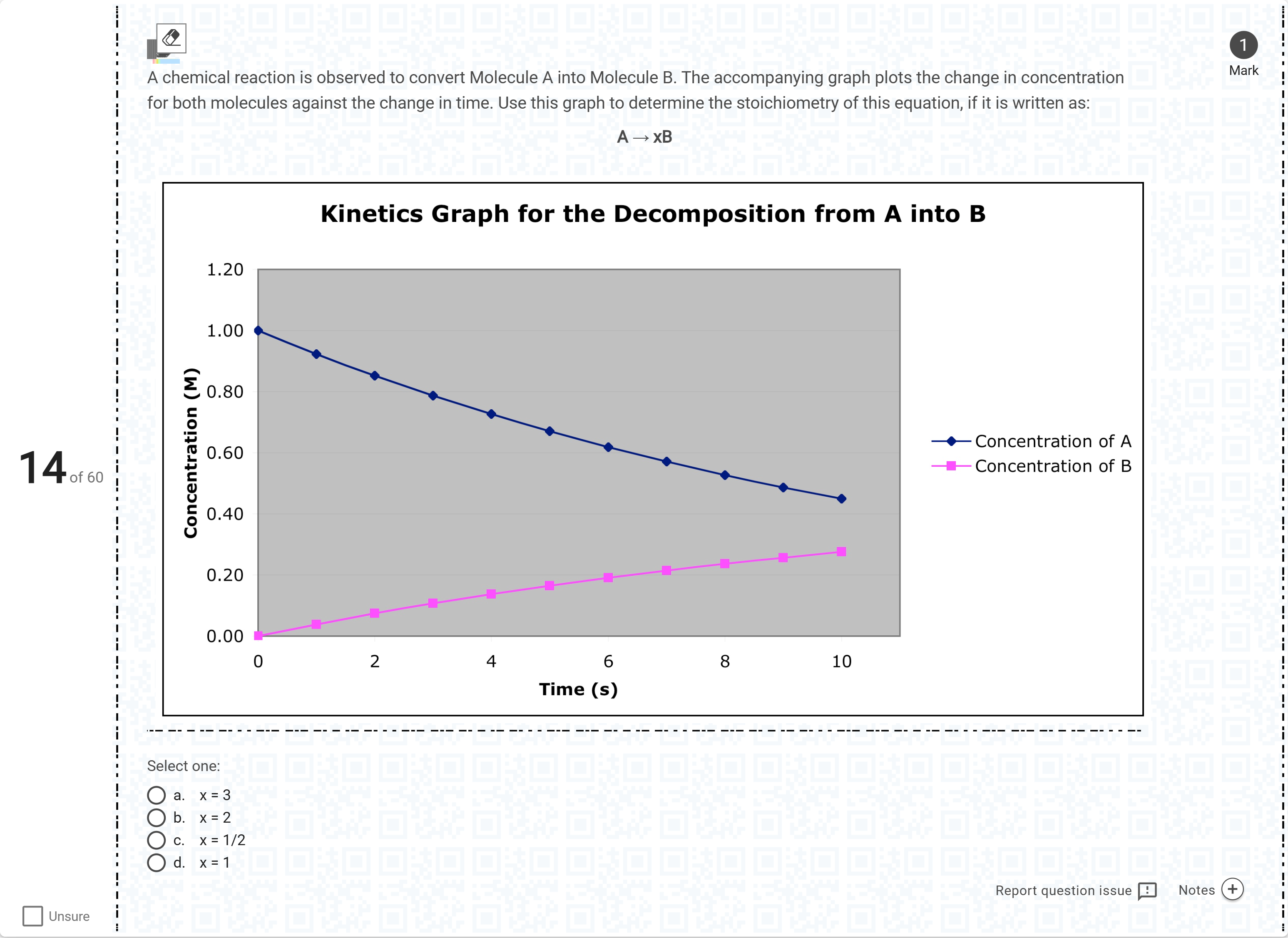
A chemical reaction is observed to convert Molecule A into Molecule B. The accompanying graph plots the change in concentration for both molecules against the change in time. Use this graph to determine the stoichiometry of this equation, if it is written as: A → xB[Fill in the blank]
Options
A.a. x = 3
B.b. x = 2
C.c. x = 1/2
D.d. x = 1
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Begin by restating the given information from the graph: A is consumed over time while B is formed, and the stoichiometry is written as A → x B.
Option a (x = 3): If x were 3, then for every 1 mole of A consumed, 3 moles of B should be produced. Checking the graph, the amount of A decreases by about 0.55 M while B increases by only abo......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Which of the following diagrams illustrates the law of multiple proportions?
If sodium hydrogen carbonate is added to a hydrochloric acid solution, it will produce gaseous carbon dioxide according to the following reaction: NaHCO3(s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g) How many grams of NaHCO3 must be added to an excess of HCl(aq) to produce 50.0 mL of CO2 at 25 °C and 0.995 atm?
Carbon monoxide and oxygen react to form carbon dioxide according to the following balanced chemical equation 2 CO(g) + O2(g) ➔ 2 CO2(g) If CO(g) and O2(g) are combined in the ratio shown in the diagram below, where the black spheres represent carbon atoms and the red spheres represent oxygen atoms, which molecules will remain when the reaction is complete?
Consider the following reaction: 4FeS2(s) + 11O2(g) → 2Fe2O3(s) + 8SO2(g) If 4.0 mol of FeS2 and 4.0 mol of O2 react, how many moles of Fe2O3(s) are produced?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!