Questions
BIOL3611.13541.202610 FA2IndFA2025- Requires Respondus LockDown Browser
Single choice
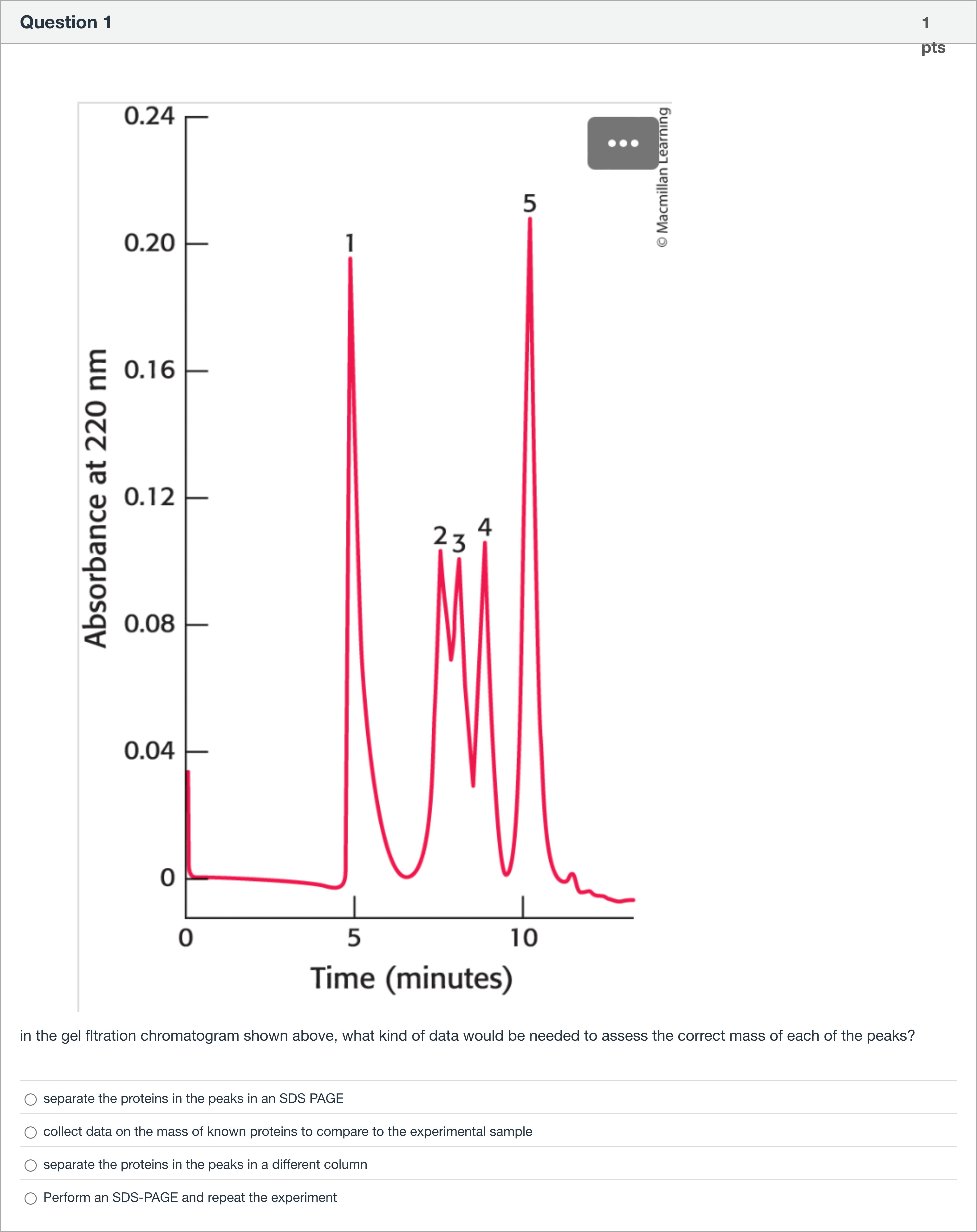
in the gel fltration chromatogram shown above, what kind of data would be needed to assess the correct mass of each of the peaks?
Options
A.separate the proteins in the peaks in an SDS PAGE
B.collect data on the mass of known proteins to compare to the experimental sample
C.separate the proteins in the peaks in a different column
D.Perform an SDS-PAGE and repeat the experiment
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To approach this question, consider what information gel filtration (size-exclusion chromatography) provides and what is required to assign a molecular weight to each peak.
Option 1: 'separate the proteins in the peaks in an SDS PAGE' — While SDS-PAGE can separate proteins by size and help identify components, using SDS-PAGE after the gel filtration run is not directly giving the masses of the peaks in the context of the chromatogram alone. It provides approxima......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
he gel filtration chromatogram shown above is from a mixture of proteins. Choose below the appropriate sizes for the peaks shown. The molecular mass is in kD.
The gel filtration chromatogram shown above is from a mixture of proteins. Choose below the appropriate sizes for the peaks shown. The molecular mass is in kD.
After finishing the purification of a DNA polymerase, researchers were surprised to find by gel filtration two peaks (70kD and 30kD) instead of one. An SDS-PAGE gel of the gel filtration fractions containing the peaks showed that indeed there were two proteins; one was identified as the DNA polymerase (30kD) and the other as a recombinase (40kD). A new purification scheme was devised that isolated the two proteins from each other as shown by gel filtration. The researchers hypothesized the proteins form a complex. The two purified proteins were mixed in a 1:1 ratio and gel filtration was used again. What would be your prediction of the gel filtration chromatography if the hypothesis were incorrect?
You wish to separate out the following mixture of proteins by size exclusion chromatography under native (non-denaturing) conditions.Protein X is is a homodimer. Each subunit is 25 kDa Protein Y is composed of three subunits that total 120 kDa; two of the subunits are linked by a disulphide bond. Protein Z is a monomer with a molecular weight of 30 kDa. What is their expected order of elution?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!