Questions
CHEM 1210 AU2025 (15738) CHEM 1210 Practice Exam #4 v2 (Ch 7.1–7.4, 8, 9.1–9.6)- Requires Respondus LockDown Browser
Single choice
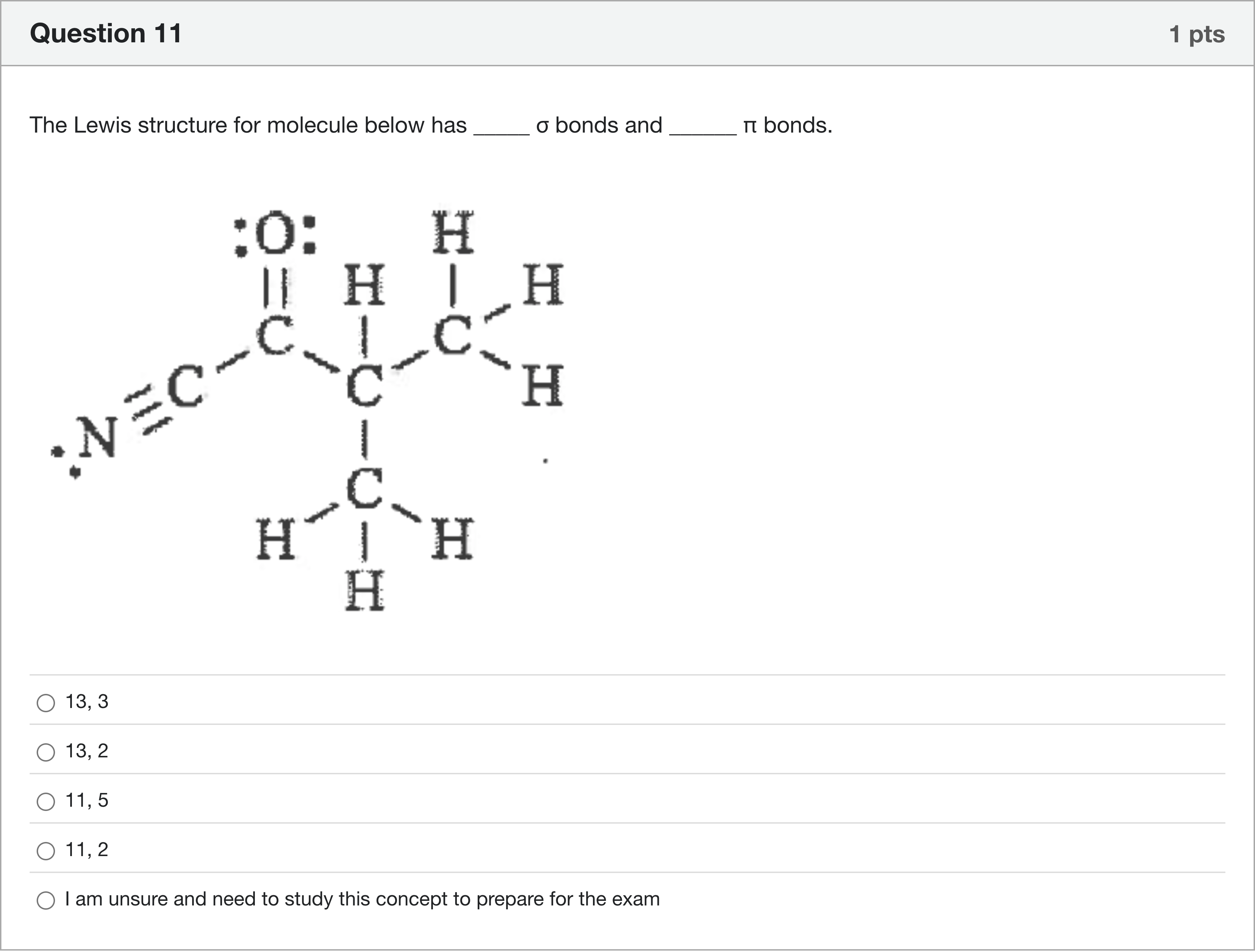
The Lewis structure for molecule below has _____ σ bonds and ______ π bonds.
Options
A.13, 3
B.13, 2
C.11, 5
D.11, 2
E.I am unsure and need to study this concept to prepare for the exam
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Question restatement: The Lewis structure for the molecule shown has _____ σ bonds and ______ π bonds. Answer options are: 1) 13, 3 2) 13, 2 3) 11, 5 4) 11, 2 5) I am unsure and need to study this concept to prepare for the exam.
Step-by-step analysis of each option:
Option 1: "13, 3". To evaluate this, count all single bonds as σ (sigma) bonds and all double or triple bonds contribute π bonds in addition to σ. In a typical organic Lewis structure with several single bonds (C–H, C–C, C–N, C–O, etc.), plus any multiple bonds (double or triple), you can accumulate a relatively large number of σ bonds from each single bond and a smaller number of π bonds from the multiple bonds. If the depicted molecule features multiple single bonds along a carbon chain and a few multiple bonds (f......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Given the Lewis structure below: There are [Blank-1] sigma bonds and [Blank-2] pi bonds.
sigma bonds are stronger than pi bonds
How many sigma and pi bonds are in the molecule shown below? Sigma bonds: 8 Pi bonds: 1
How many sigma and pi bonds are in the molecule shown below? Sigma bonds: [ Select ] 3 6 8 2 9 4 5 7 0 1 10 Pi bonds: [ Select ] 9 5 7 10 3 6 4 2 0 8 1
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!