Questions
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 21 Quiz C09-3
Multiple dropdown selections
How many sigma and pi bonds are in the molecule shown below? Sigma bonds: [ Select ] 3 6 8 2 9 4 5 7 0 1 10 Pi bonds: [ Select ] 9 5 7 10 3 6 4 2 0 8 1
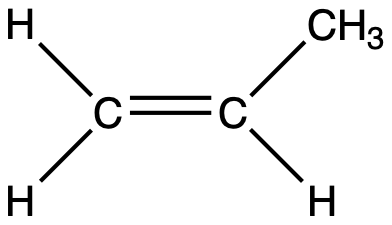
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To determine the number of sigma and pi bonds in a molecule, we need to inspect the molecular diagram or formula to count bonds between atoms and to identify multiple bonds.
Step 1: Clarify what counts as a sigma bond vs a pi bond.
- Each bond between two atoms consists of at least one sigma (σ) bond. In a single bond, there is exactly one σ bond and no π bonds.
- In a double bond, there is one σ bond and one π bond.
- In a triple bond, there is one σ bond and two π bonds.
- So, the total number of σ bonds is the count of all bonds, where multiple bonds contribute exactly one σ per bond type (double or triple), plus any single bonds.
Step 2: How to count from the dia......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!