Questions
CHEM0005_24-25 CHEM0005 2024-25 On-line Exam
Multiple fill-in-the-blank
Question textThe figure shows a series of substituted anilines A-F, each with a different R-group in the para position to the NH2. For each molecule, indicate the electronic effect that the R group has on the benzene ring based on mesomeric and inductive effects. CH3 is known to be inductively electron donating, but has no mesomeric contribution. [table] Compound | R group | Mesomeric Effect | Inductive Effect A | NO2 | Answer 1 Question 54 Electron WithdrawingElectron DonatingNone | Answer 2 Question 54 Electron WithdrawingElectron DonatingNone B | OMe | Answer 3 Question 54 Electron WithdrawingElectron DonatingNone | Answer 4 Question 54 Electron WithdrawingElectron DonatingNone C | NMe3+ | Answer 5 Question 54 Electron WithdrawingElectron DonatingNone | Answer 6 Question 54 Electron WithdrawingElectron DonatingNone D | CH3 | None | Electron Donating E | H | Answer 7 Question 54 Electron WithdrawingElectron DonatingNone | Answer 8 Question 54 Electron WithdrawingElectron DonatingNone F | CF3 | Answer 9 Question 54 Electron WithdrawingElectron DonatingNone | Answer 10 Question 54 Electron WithdrawingElectron DonatingNone [/table]
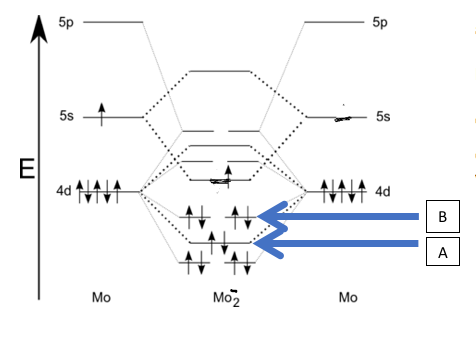
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
We are asked to indicate for each para substituent R the electronic effect on the benzene ring based on mesomeric (resonance) and inductive effects.
First, recall general rules:
- Electron-donating via mesomeric (R groups with lone pairs or π-donation) increase electron density in the ring (activating groups for electrophilic substitution in o/p positions).
- Electron-withdrawing via mesomeric effects pull electron density from the ring through resonance (often deactivating, or at least altering reactivity).
- Inductive effects reflect through-bond withdrawal or donation based on electronegativity and polarizability; strong electronegative substituents withdraw electron densit......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
People born without creativity never can develop it
A product has a selling price of $20, a contribution margin ratio of 40% and fixed cost of $120,000. To make a profit of $30,000. The number of units that must be sold is: Type the number without $ and a comma. Eg: 20000
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!