Questions
My LMS Subjects Practice LMS questions, Week 3
Multiple fill-in-the-blank
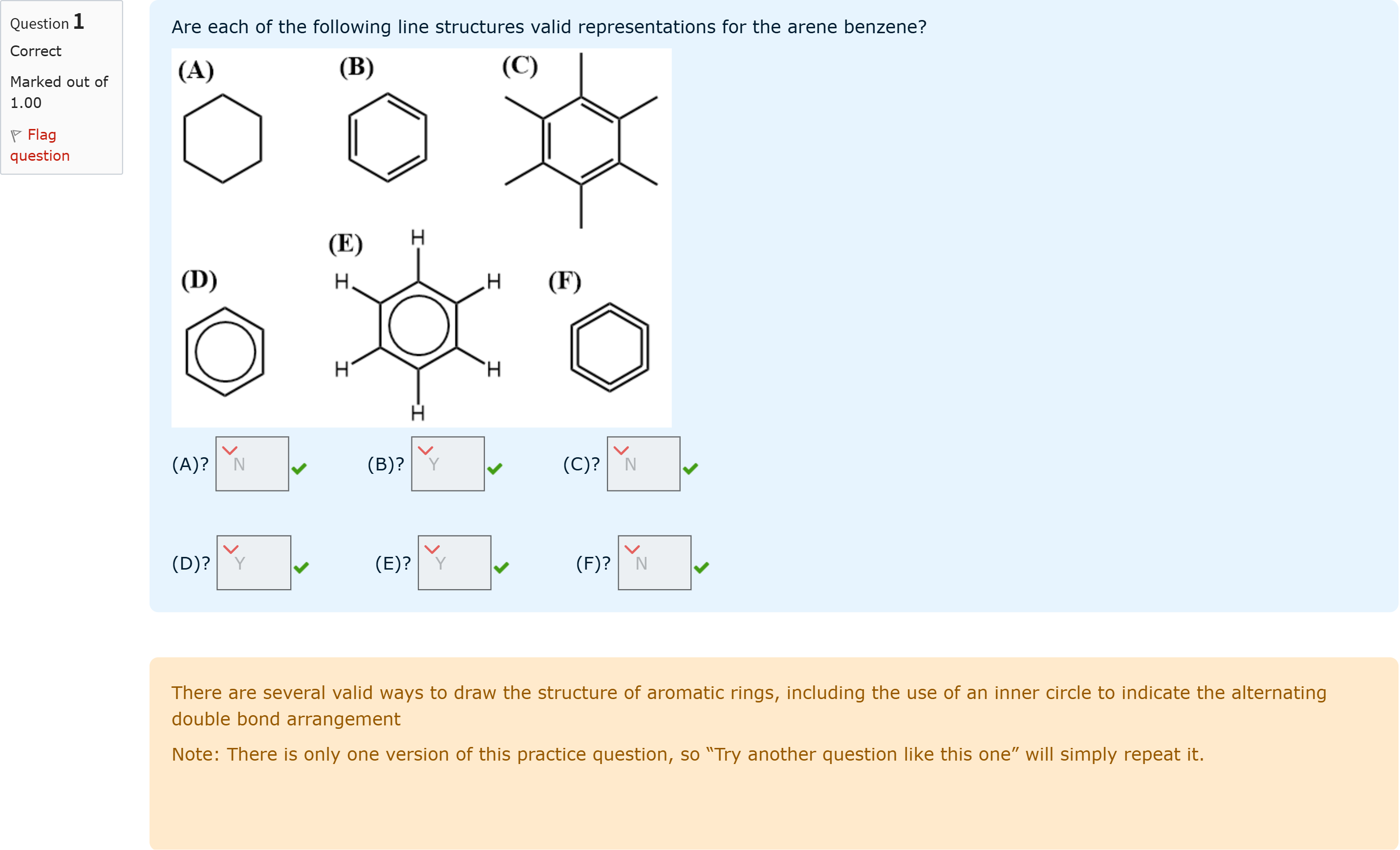
Question textAre each of the following line structures valid representations for the arene benzene?(A)? Answer 1 Question 1[select: , Y, N] (B)? Answer 2 Question 1[select: , Y, N] (C)? Answer 3 Question 1[select: , Y, N](D)? Answer 4 Question 1[select: , Y, N] (E)? Answer 5 Question 1[select: , Y, N] (F)? Answer 6 Question 1[select: , Y, N]
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks which of the listed line structures are valid representations for the arene benzene. Below, I examine each option in turn, noting typical conventions used to depict benzene and common pitfalls.
(A) This structure appears to be a six-membered ring with all single bonds and no circle or alternating pattern indicated. A pure cyclohexane-like ring (no aromatic characteristics) is not benzene, which is an aromatic ring with delocalized electrons. Therefore, this representation is not a valid depiction of benzene, because it fails to convey aromaticity and the characteristic delocalized electron system.
......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
People born without creativity never can develop it
A product has a selling price of $20, a contribution margin ratio of 40% and fixed cost of $120,000. To make a profit of $30,000. The number of units that must be sold is: Type the number without $ and a comma. Eg: 20000
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!