Questions
MCD4400 Chemistry II - Trimester 3 - 2025
Single choice
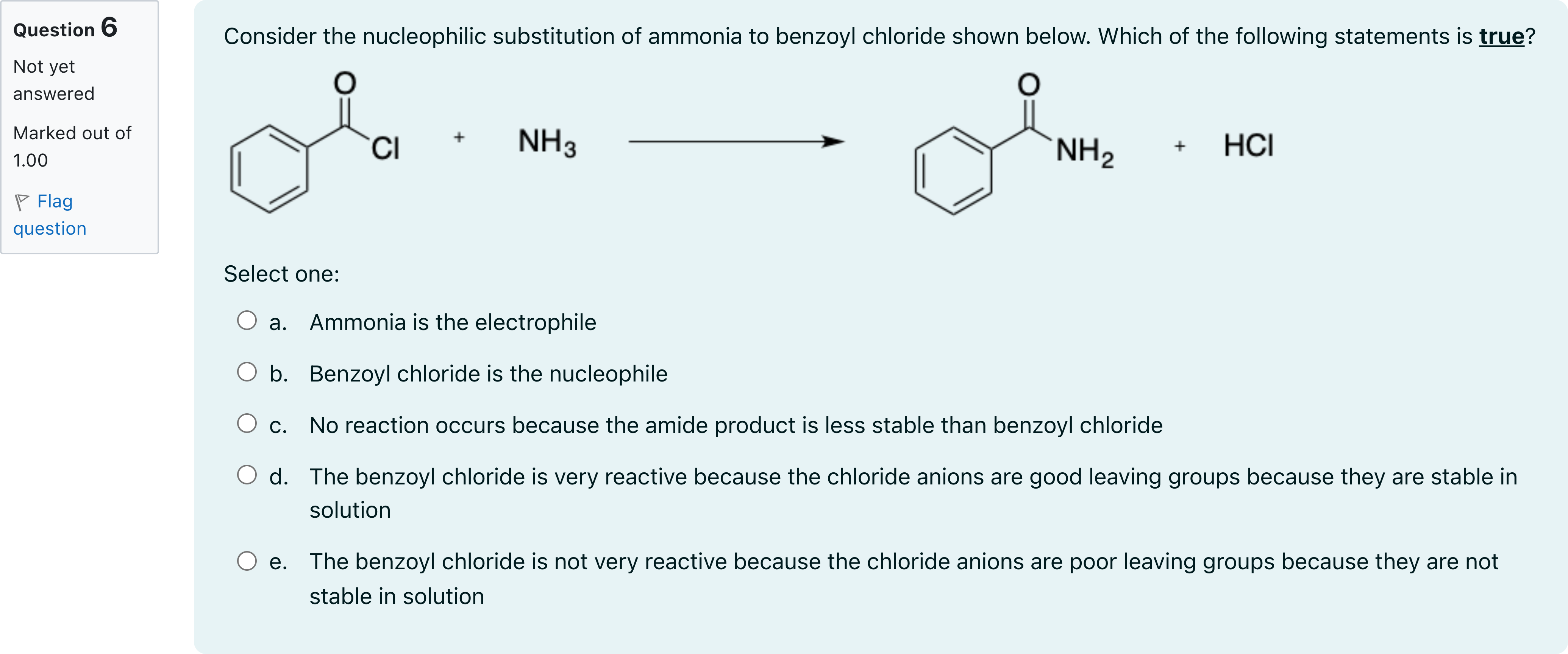
Consider the nucleophilic substitution of ammonia to benzoyl chloride shown below. Which of the following statements is true?
Options
A.a. Ammonia is the electrophile
B.b. Benzoyl chloride is the nucleophile
C.c. No reaction occurs because the amide product is less stable than benzoyl chloride
D.d. The benzoyl chloride is very reactive because the chloride anions are good leaving groups because they are stable in solution
E.e. The benzoyl chloride is not very reactive because the chloride anions are poor leaving groups because they are not stable in solution
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Question: Consider the nucleophilic substitution of ammonia to benzoyl chloride shown below. Which of the following statements is true?
Option a: Ammonia is the electrophile. In this reaction, ammonia acts as the nucleophile, attacking the carbonyl carbon of benzoyl chloride. The electrophilic center is the carbonyl carbon of benzoyl chloride, not the ammonia molecule. Therefore this statement is incorrect.
Option b: Benzoyl chloride is ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Compound (I) reacts with methanol to produce intermediate (II) and chloride ions. Which one of the following mechanisms is correct?
Consider the reaction of ethanoyl chloride with methanol shown below. What product(s) may you expect to be formed from the reaction?
Consider the nucleophilic addition of ammonia to benzoyl chloride shown below. Which of the following statements is true?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!