Questions
My LMS Subjects Quiz 4
Numerical
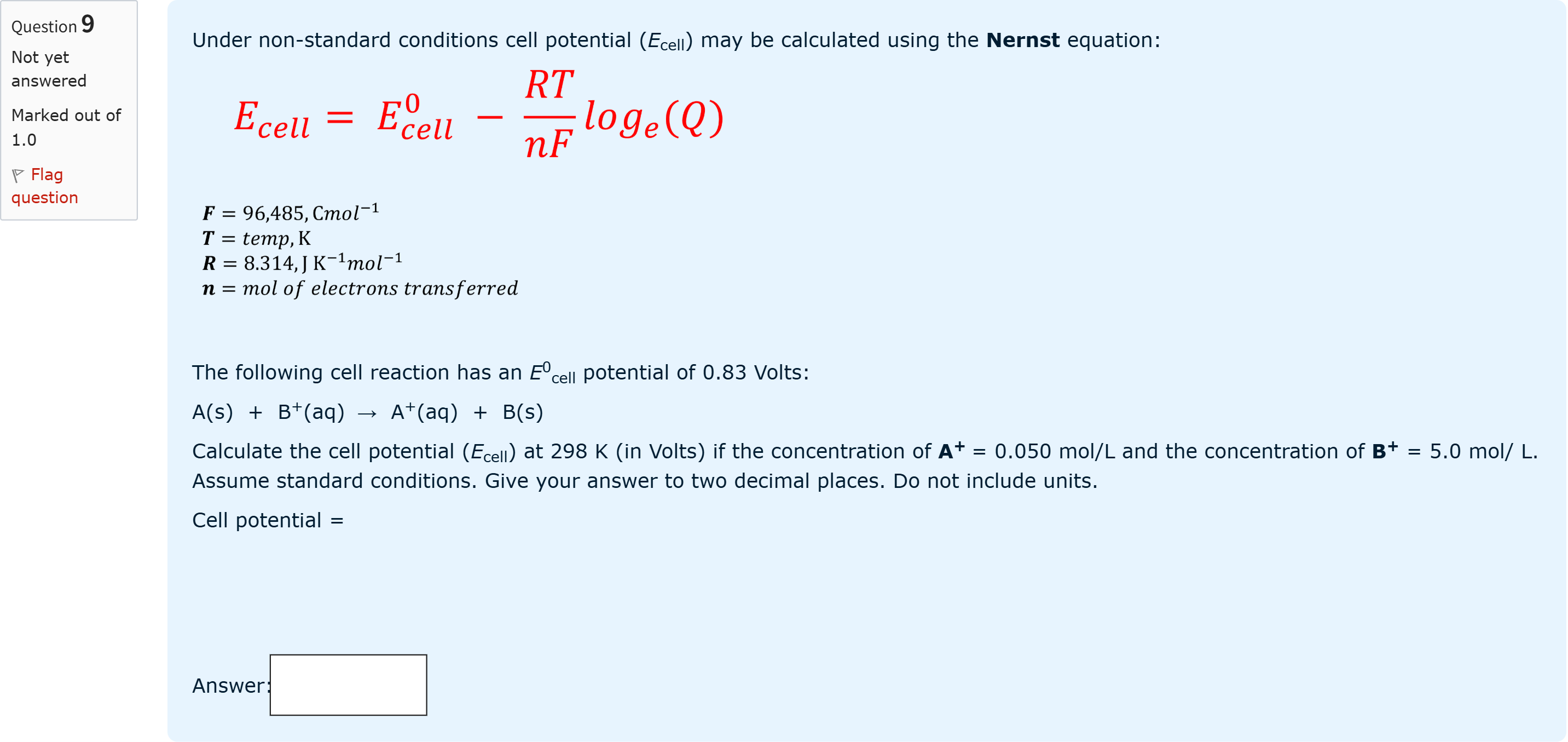
Under non-standard conditions cell potential (Ecell) may be calculated using the Nernst equation: The following cell reaction has an E0cell potential of 0.83 Volts: A(s) + B+(aq) → A+(aq) + B(s) Calculate the cell potential (Ecell) at 298 K (in Volts) if the concentration of A+ = 0.050 mol/L and the concentration of B+ = 5.0 mol/ L. Assume standard conditions. Give your answer to two decimal places. Do not include units. Cell potential =
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Start by identifying the given information and the formula to use. The problem provides E°cell = 0.83 V, T = 298 K, n = 1, and the reaction A(s) + B+(aq) → A+(aq) + B(s). The Nernst equation in the form given by the image uses the natural logarithm: Ecell = E°cell − (RT / (nF)) ln(Q). We need to determine Q from the concentrations of the aqueous species involved. In this reaction, solids A a......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Under non-standard conditions cell potential (Ecell) may be calculated using the Nernst equation: The following cell reaction has an E0cell potential of 0.83 Volts: A(s) + 3B+(aq) → A3+(aq) + 3B(s) Calculate the cell potential (Ecell) at 60 oC (in Volts) if the concentration of A+ = 4.0 mol/L and the concentration of B+ = 0.001 mol/ L. Assume standard conditions. Give your answer to two decimal places... This one is a little trickier! Cell potential =
The equilibrium constant of the reaction shown below is 2.41 × 108 at 25°C: 3Ag(s) + NO3—(aq) +4H+(aq) → 3Ag+(aq) + NO(g) + 2H2O(l) Calculate the value of E°cell for a cell utilizing this reaction.
Consider the following voltaic cell: Ni(s)Ni2+(aq)Cl2(g)Cl–(aq)Pt(s) If the standard potentials of the Ni2+/Ni and Cl2/Cl– couples are –0.23 V and +1.36 V, respectively, at 25 °C calculate the voltage of this cell when {Ni2+] = 0.100 M, [Cl–] = 0.100 M, and the pressure of Cl2(g) = 1.50 atm.
Consider the following voltaic cell: Ni(s)∣Ni2+(aq)∥Cl2(g)∣Cl–(aq)∣Pt(s) If the standard potentials of the Ni2+/Ni and Cl2/Cl– couples are –0.23 V and +1.36 V, respectively, at 25 °C calculate the voltage of this cell when {Ni2+] = 0.100 M, [Cl–] = 0.100 M, and the pressure of Cl2(g) = 1.50 atm.
More Practical Tools for International Students
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!