Questions
CHM1051 MUM S2 2025 CHM1051 Practice Exam 1
Single choice
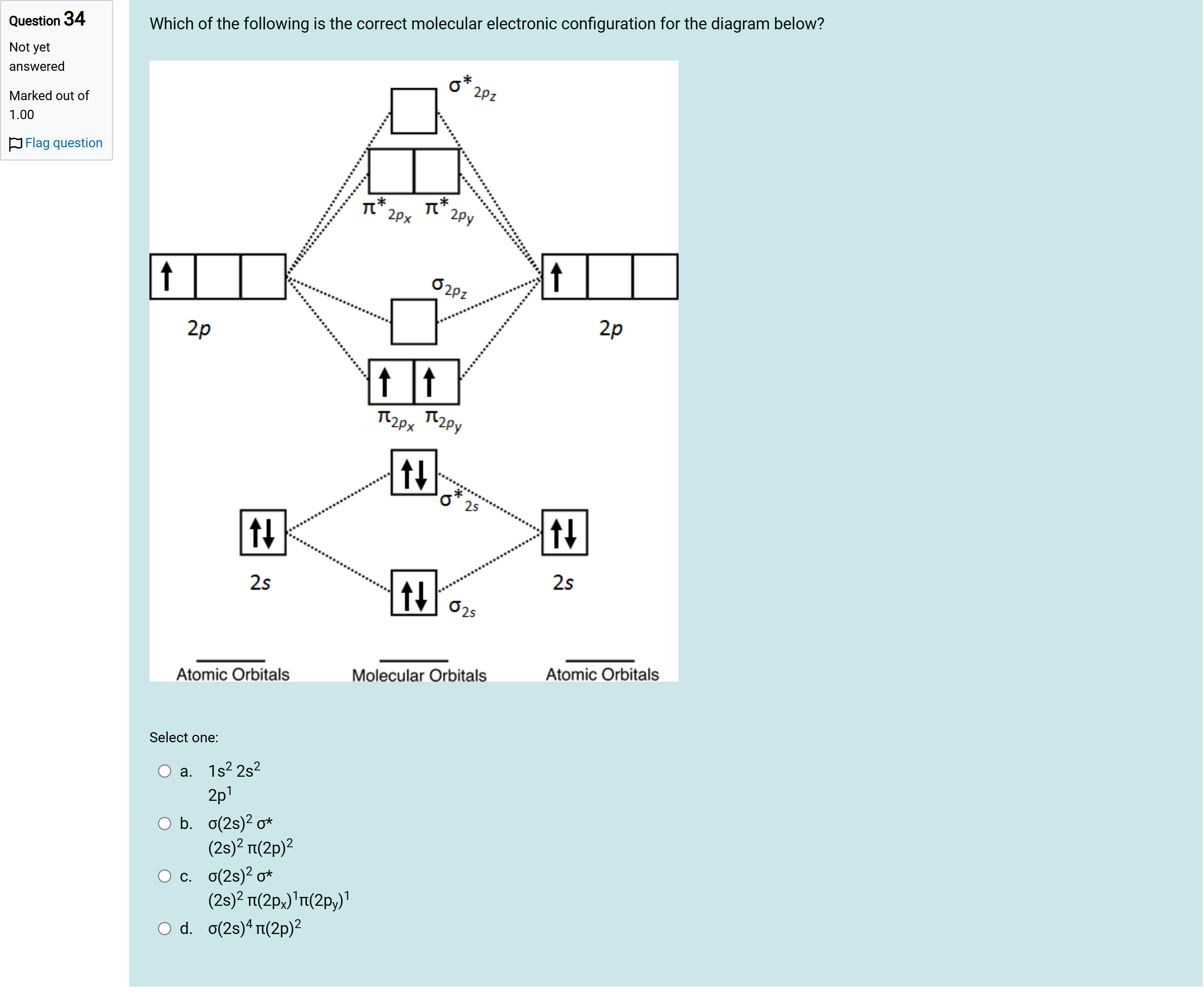
Which of the following is the correct molecular electronic configuration for the diagram below?
Options
A.a. 1s2 2s2 2p1
B.b. σ(2s)2 σ*(2s)2 π(2p)2
C.c. σ(2s)2 σ*(2s)2 π(2px)1π(2py)1
D.d. σ(2s)4 π(2p)2
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
First, I will restate the question and the available choices to set the context for analysis.
Question: Which of the following is the correct molecular electronic configuration for the diagram below?
Options:
- a. 1s2 2s2 2p1
- b. σ(2s)2 σ*(2s)2 π(2p)2
- c. σ(2s)2 σ*(2s)2 π(2px)1π(2py)1
- d. σ(2s)4 π(2p)2
Now, let’s examine each option in relation to the diagram provided (which shows electrons in σ(2s), σ*(2s), and the two degenerate π orbitals π(2px) and π(2py)).
Option a: 1s2 2s2 2p1
- This ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Using the molecular orbital theory, predict how many unpaired electrons are in the B22– ion.
Using molecular orbital theory, predict the bond order for the O2+ ion.
Assuming an MO diagram with orbital energies like that of O2 what is the bond order and number of unpaired electrons for the molecule NO2- ?
Using the molecular orbital theory, predict how many unpaired electrons are in the O2+ ion.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!