Questions
Multiple dropdown selections
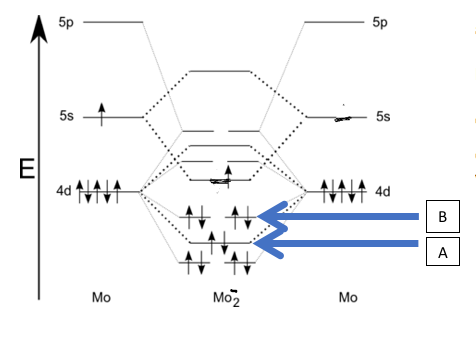
Though we haven’t looked at anything but a few particular MO diagrams, you can extrapolate what you have learned to other diagrams which include d orbitals. Use the diagram shown below, and what you know from doing MO diagrams of 2nd row diatomics to answer the following questions. Is the ion shown paramagnetic or diamagnetic? [ Select ] diamagentic paramagnetic What is the name of the orbital labelled A? [ Select ] sigma 4p pi 4d sigma 4d* pi 4d* sigma 4s pi 4p sigma 4d sigma4s* sigma 4p* What is the bond order? [ Select ] 3.5 5.5 2 4.5 6 1.5 4 3 2.5 5
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
We will examine each part of the question separately, using the MO diagram context shown.
First, restating the question and options:
- Is the ion shown paramagnetic or diamagnetic? Options: diamagnetic, paramagnetic
- What is the name of the orbital labeled A? Options include several sigma/pi and 4s/4p/4d variants with and without asterisks indicating antibonding
- What is the bond order? Options include several fractional and whole numbers such as 3.5, 2.5, 2, 4, etc.
Now, evaluate each option:
1) Is the ion paramagnetic or diamagnetic?
- Diamagnetic option: This would be true if every electron were paired in the molecular orbitals. If the MO diagram shows any unpaired electrons in the d, p, or other orbitals, this would contradict a diamagnetic assignment.
- Paramagnetic option: This is chosen if there are unpaired electrons in the MO configuration, which would produce a magnet......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Using the molecular orbital theory, predict how many unpaired electrons are in the B22– ion.
Using molecular orbital theory, predict the bond order for the O2+ ion.
Which of the following is the correct molecular electronic configuration for the diagram below?
Assuming an MO diagram with orbital energies like that of O2 what is the bond order and number of unpaired electrons for the molecule NO2- ?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!