Still overwhelmed by exam stress? You've come to the right place!
We know exam season has you totally swamped. To support your studies, access Gold Membership for FREE until December 31, 2025! Normally £29.99/month. Just Log In to activate – no strings attached.
Let us help you ace your exams efficiently!
Questions
CHEM 1210 AU2025 (15738) Exam 4- Requires Respondus LockDown Browser
Single choice
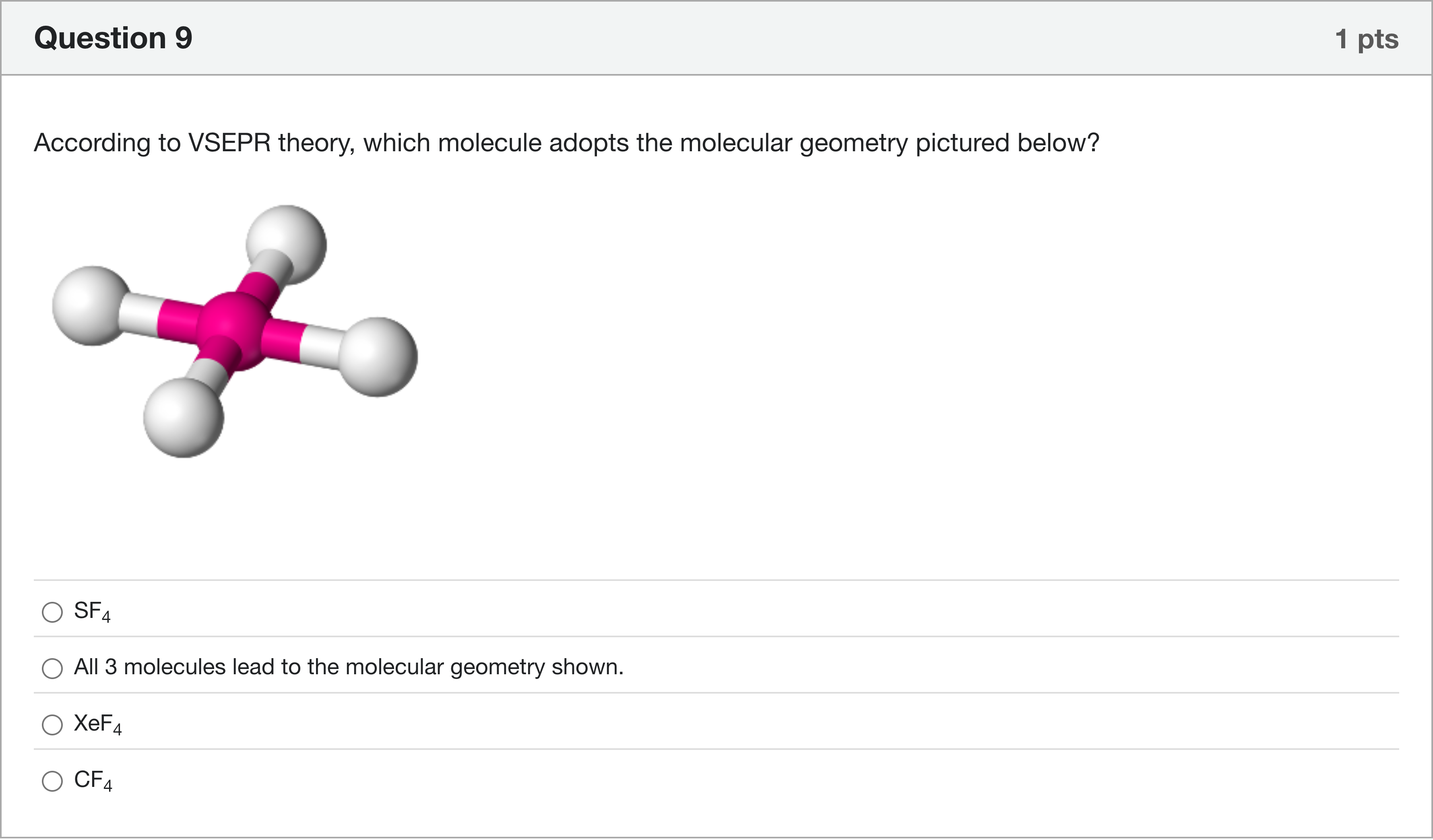
According to VSEPR theory, which molecule adopts the molecular geometry pictured below? 14A
Options
A.SF4
B.All 3 molecules lead to the molecular geometry shown.
C.XeF4
D.CF4
View Explanation
Standard Answer
Please login to view
Approach Analysis
To determine which molecule adopts the pictured geometry, we need to compare the VSEPR shapes produced by different electron arrangements around the central atom.
Option SF4: This molecule has one lone pair on sulfur in addition to the four S–F bonds. The presence of a lone......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
More Practical Tools for International Students
Making Your Study Simpler
To make preparation and study season easier for more international students, we've decided to open up Gold Membership for a limited-time free trial until December 31, 2025!